Polyvinylpyrrolidone affects thermal stability of drugs in solid dispersions
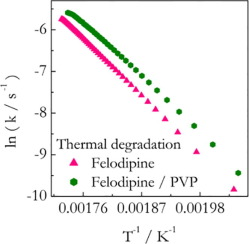
The present study explores the hypothesis that a polymer can affect the thermal stability of a drug in solid polymer-drug dispersions. The hypothesis is tested in a systematic fashion by combining isoconversional kinetic analysis with thermogravimetric measurements on several solid dispersions. Experimental systems involve three drugs: indomethacin (IMC), felodipine (FD), and nifedipine (ND) and their solid dispersions with polyvinylpyrrolidone (PVP). It is found that PVP stabilizes IMC but destabilizes FD and ND. Isoconversional kinetic analysis provides insights into the origin of the observed effects. The enhanced thermal stability of IMC in the PVP matrix is associated with an increase in the activation energy of the respective degradation process. A detrimental effect of the PVP matrix on the stability of FD and ND has been linked to a decrease in the activation energy and an increase in the preexponential factor respectively. The molecular underpinnings of the observed effects are discussed. It is concluded that the effects in question are of relevance for drug performance and need to be taken into account in preformulation studies.