Solubilization of drugs using sodium lauryl sulfate: Experimental data and modeling
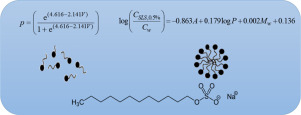
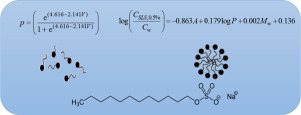
Micellar solubilization is a great method for increasing drugs solubility in aqueous environments. At concentrations above the critical micelle concentration (CMC), micelles are formed and they are able to increase the apparent aqueous solubility of poorly soluble drugs. Sodium lauryl sulfate (SLS) is one of the common solubilizing agents in pharmaceutical sciences. Investigation on the water solubility of drugs in the presence of surfactants and the development of a relationship between drug solubility in the presence of SLS and structural descriptors is an important issue in the prediction and understanding of the solubilization mechanism. The aims of this study are: determination of experimental solubility of drugs in the presence of SLS and development of models for finding a relationship between solubilization factor by SLS and structural descriptors. Samples were prepared by adding excess amount of 19 drugs (with diverse structural and physicochemical properties) to water and an aqueous solutionof SLS at different concentrations, that is, less than (0.1%) and above the CMC (0.5%). The mixtures were placed in a shaker-incubator for 72–96 h at 37 °C. Then, the equilibrated samples were filtered and analyzed at maximum wavelengths by UV-spectrophotometry and the concentrations were calculated based on the calibration curves. Afterward, the molecular descriptors of drugs were computed and their relationship with solubilization factor in the presence of SLS was investigated. Most of the drugs showed a considerable increase in solubility above the CMC (0.5%) of SLS. Therefore, the effective mechanism for solubilization by surfactants is the formation of micelles. On the other hand, a good correlation was observed between structural descriptors and solubilization power in the presence of surfactant. Overall, SLS is a good solubilization agent and the solubility in aqueous solution of SLS depends on various structural descriptors.