- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
04. October 2018
Praziquantel is an antiparasitic drug used for decades. Currently, the praziquantel commercial preparation is a racemic mixture, inwhich only the levo-enantiomer possesses anthelmintic activity. The knowledge of its properties in the solid state and other chemical-physical properties is necessary for improving its efficacy andapplications. Drug solid dispersions were prepared with calcium carbonate at 1:5 drug to excipient weight ratio by solventevaporation method. Then, the modification of the...
30. August 2018
This study aimed to improve dissolution rate of valsartan in an acidic environment and consequently its oral bioavailability by solid dispersion formulation. Valsartan was selected as a model drug due to its low oral bioavailability (~23%) caused by poor solubility of this drug in the low pH region of gastrointestinal tract (GIT) and presence of absorption window in the upper part of GIT. Solid dispersions were prepared by solvent evaporation method with Eudragit® E100, Soluplus® or...
30. July 2018
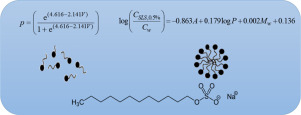
Micellar solubilization is a great method for increasing drugs solubility in aqueous environments. At concentrations above the critical micelle concentration (CMC), micelles are formed and they are able to increase the apparent aqueous solubility of poorly soluble drugs. Sodium lauryl sulfate (SLS) is one of the common solubilizing agents in pharmaceutical sciences. Investigation on the water solubility of drugs in the presence of surfactants and the development of a relationship between drug...
16. June 2018
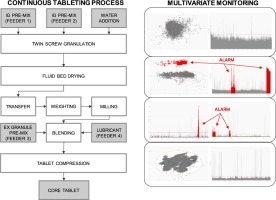
The pharmaceutical industry is undergoing a significant change in product development and manufacturing strategies with the progressive shift from batch to continuous processes. These typically feature vast volumes of data generated by the numerous sensors connected to several unit operations running over the period of several hours or even days and that demand the application of increasingly efficient tools for process understanding, monitoring and control. This paper describes the use of...
18. October 2017
In the drug delivery area, versatile therapeutic systems intended to yield customized combinations of drugs, drug doses and release kinetics have drawn increasing attention, especially because of the advantages that personalized pharmaceutical treatments would offer.
28. September 2017
A comprehensive model with all effective phenomena in drug release such as diffusion, swelling and erosion was considered. In this work, a mathematical model was developed to describe drug release from controlled release HPMC matrices as a favorable system in pharmaceutical industries. As a novel study, the impact of the MCC presence as a filler in tablet preparation process was considered in the mathematical model. In addition, we found that the volume expansion of these polymeric matrices did...
12. April 2017
Abstract Disintegrants are used as excipients to ensure rapid disintegration of pharmaceutical tablets and further ensure proper dissolution of the active pharmaceutical ingredient. This study investigates, disintegration mechanisms of chitin and common disintegrants. Swelling assessment (swelling force and swelling ratio) in different media; compaction behavior (pure or mixed with other excipients) tabletability, deformation (Heckel modeling) and compact disintegration times were investigated...
26. March 2017
Abstract In this work, amorphous paracetamol/Eudragit® formulations for four Eudragit® (polymeric excipients) were prepared by spray drying technique. The simultaneous dissolution kinetics of paracetamol and Eudragit® from these formulations were measured as function of pH in vitro using a rotating disk system (USP II). Paracetamol dissolution mechanisms were analyzed by comparing the dissolution rates of paracetamol and excipient. It was found that a controlled paracetamol dissolution was...
24. March 2017
ABSTRACT Introduction: For almost two decades there has been intense debate about whether the amorphous solid state form could resolve the solubility problems and subsequent bioavailability issues of many small molecule drugs. Since the amorphous form is a high energy and unstable state of solid matter, any material in that form requires stabilization. Areas covered: This review examines the technologies being exploited to stabilize the amorphous state in co-amorphous formulations. The review...
27. February 2017
Abstract Continuous manufacturing has attracted increasing research attention in the pharmaceutical industry within the last decade. Based on the extensive experimental studies, numerous modeling and computational approaches have been developed to capture the process information and make predictions. Moreover, flowsheet models have been built to simulate the dynamic behaviors of a plant-wide manufacturing process with respect to different process input factors. However, there still lacks a...