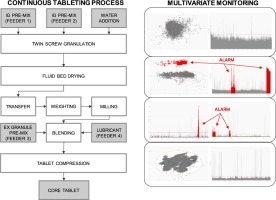
The pharmaceutical industry is undergoing a significant change in product development and manufacturing strategies with the progressive shift from batch to continuous processes. These typically
feature vast volumes of data generated by the numerous sensors connected to several unit operations running over the period of several hours or even days and that demand the application of
increasingly efficient tools for process understanding, monitoring and control. This paper describes the use of multivariate statistical process modeling by means of chemometric methods to monitor
the continuous wet granulation tableting process for a drug product currently under development. Models are tailored to the different units that make up the continuous tableting line, from material
feeding and granulation up to tablet compression, where the solutions devised reflect the different dynamics of each unit and are used as maintenance and intervention tools to optimise manufacturing
and associated operations retrospectively as well as in real-time, as part of the product industrialisation programme.