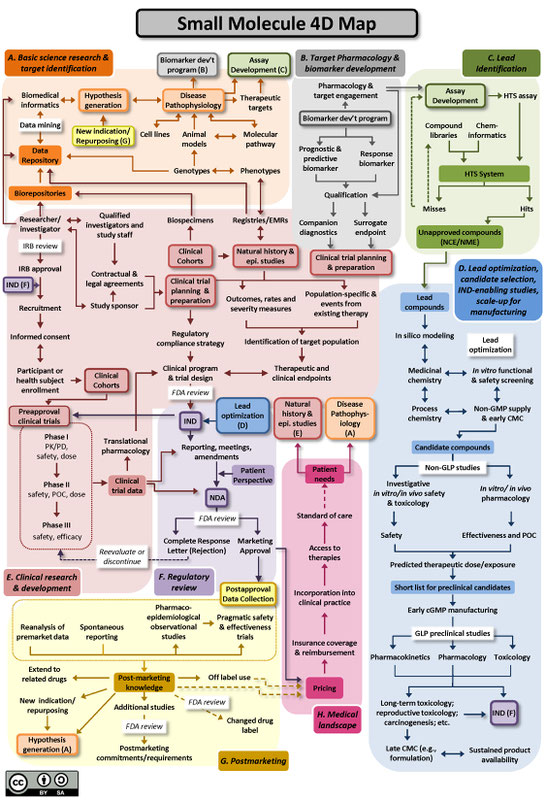
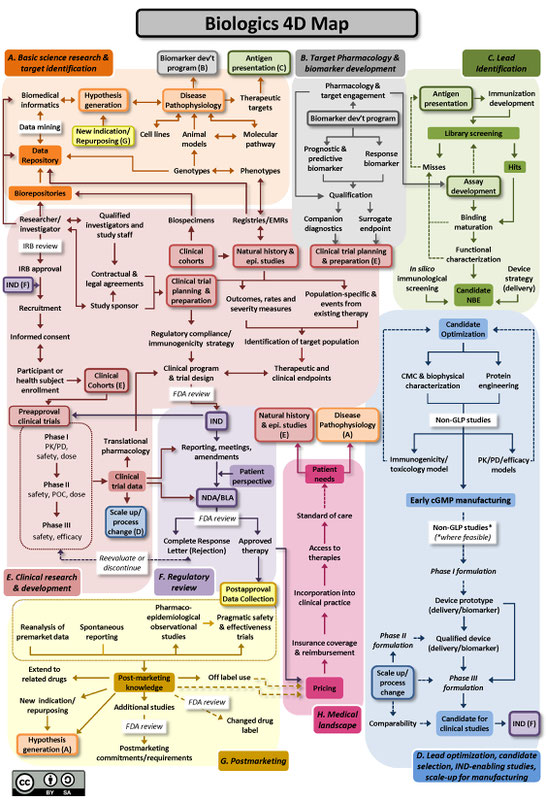
The Drug Discovery, Development and Deployment Maps (4DM) provide dynamic representations of the modern therapeutic development process to more easily identify inefficiencies and integrate efforts to expedite new therapies for patients. The maps provide a common framework for discussing the therapeutic development process and serve as an education tool for those who are new to it. A common language and collective knowledge base for therapeutic development is essential to enable systemwide improvements that will benefit patients. The 4DM can help facilitate dialogue among those interested or participating in drug development to explore innovative solutions to existing bottlenecks and potential collaborative action to overcome those barriers and accelerate new medicine discovery.
Two versions of the 4DM are available below: one for small molecules (Figure A), and another for biologics, using monoclonal antibodies as the representative therapeutic (Figure B). Small molecule drugs are chemically manufactured compounds of relatively low molecular size that make up the vast majority of drugs on the market today. Biologic drugs are large complex molecules manufactured by a living system, such as a microorganism, or plant or animal cells, and include antibodies and vaccines. These two maps illustrate some of the unique differences between the development of biological drugs and that of more traditional small molecule therapeutics. Both files are licensed to the public under the Creative Commons Attribution-Share Alike International 4.0 (CC BY-SA 4.0) license, which allows use and adaption as long as the user provides attribution and shares any adaptations back to the public under the same license.
The 4DM were developed by members of an Action Collaborative of the Forum on Drug Discovery, Development, and Translation (the Forum) of the National Academies of Sciences, Engineering, and Medicine. The Forum includes leaders from private sector sponsors of biomedical and clinical research; federal agencies sponsoring and regulating biomedical and clinical research; foundations; the academic community; consumers; and federal and private health plans. NCATS Director Dr. Christopher P. Austin is a member of the Forum and served as co-lead of the Action Collaborative.
Recommended for you