- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
07. July 2018
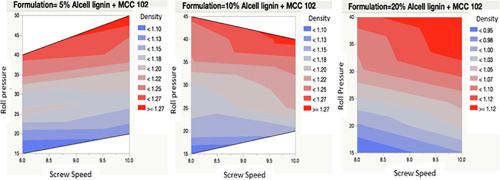
In this study, a process map was developed in an effort to improve the understanding of dry granulation of pharmaceutical excipients by roll compaction process, and to implement the quality-by-design (QbD) approach. Through development of the process map, a correlation was made between the critical process parameters (roll pressure, screw speed), and critical quality attributes (density of ribbons and granule size). This method reduces development time, quantity of materials required and cost....
27. December 2017
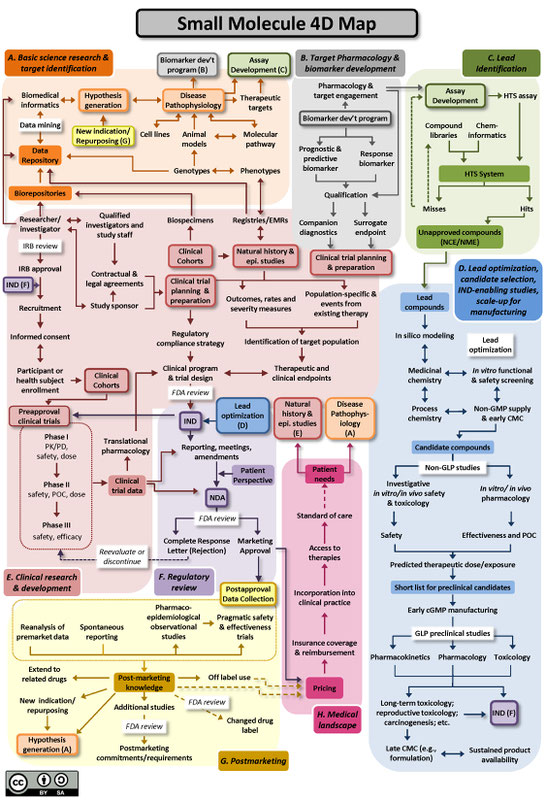
The Drug Discovery, Development and Deployment Maps (4DM) provide dynamic representations of the modern therapeutic development process to more easily identify inefficiencies and integrate efforts to expedite new therapies for patients. The maps provide a common framework for discussing the therapeutic development process and serve as an education tool for those who are new to it.