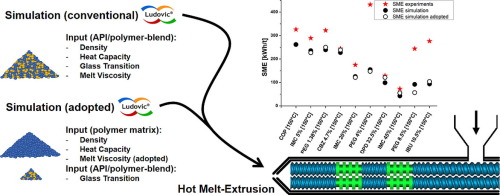
Simulation of HME processes is a valuable tool for increased process understanding and ease of scale-up. However, the experimental determination of all required input parameters is tedious, namely the melt rheology of the amorphous solid dispersion (ASD) in question. Hence, a procedure to simplify the application of hot-melt extrusion (HME) simulation for forming amorphous solid dispersions (ASD) is presented. The commercial 1D simulation software Ludovic® was used to conduct (i) simulations using a full experimental data set of all input variables including melt rheology and (ii) simulations using model-based melt viscosity data based on the ASDs glass transition and the physical properties of polymeric matrix only. Both types of HME computation were further compared to experimental HME results. Variation in physical properties (e.g. heat capacity, density) and several process characteristics of HME (residence time distribution, energy consumption) among the simulations and experiments were evaluated. The model-based melt viscosity was calculated by using the glass transition temperature (Tg) of the investigated blend and the melt viscosity of the polymeric matrix by means of a Tg-viscosity correlation. The results of measured melt viscosity and model-based melt viscosity were similar with only few exceptions, leading to similar HME simulation outcomes. At the end, the experimental effort prior to HME simulation could be minimized and the procedure enables a good starting point for rational development of ASDs by means of HME. As model excipients, Vinylpyrrolidone-vinyl acetate copolymer (COP) in combination with various APIs (carbamazepine, dipyridamole, indomethacin, and ibuprofen) or polyethylene glycol (PEG 1500) as plasticizer were used to form the ASDs.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact