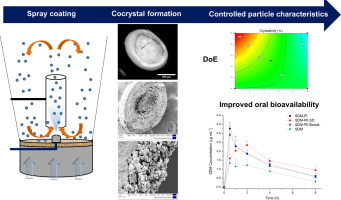
Engineering of pharmaceutical cocrystals is an advantageous alternative to salt formation for improving the aqueous solubility of hydrophobic drugs. Although, spray drying is a well-established scale-up technique in the production of cocrystals, several issues can arise such as sublimation or stickiness due to low glass transition temperatures of some organic molecules, making the process very challenging. Even though, fluidised bed spray coating has been successfully employed in the production of amorphous drug-coated particles, to the best of our knowledge, it has never been employed in the production of cocrystals. The feasibility of this technique was proven using three model cocrystals: sulfadimidine (SDM)/4-aminosalicylic acid (4ASA), sulfadimidine/nicotinic acid (NA) and ibuprofen (IBU)/ nicotinamide (NAM). Design of experiments were performed to understand the critical formulation and process parameters that determine the formation of either cocrystal or coamorphous systems for SDM/4ASA. The amount and type of binder played a key role in the overall solid state and in vitro performance characteristics of the cocrystals. The optimal balance between high loading efficiencies and high degree of crystallinity was achieved only when a binder: cocrystal weight ratio of 5:95 or 10:90 was used. The cocrystal coated beads showed an improved in vitro-in vivo performance characterised by: (i) no tendency to aggregate in aqueous media compared to spray dried formulations, (ii) enhanced in vitro activity (1.8-fold greater) against S. aureus, (iii) larger oral absorption and bioavailability (2.2-fold higher Cmax), (iv) greater flow properties and (v) improved chemical stability than cocrystals produced by other methods derived from the morphology and solid nature of the starter cores.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact