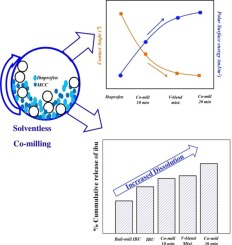
The wetting and dissolution of a BCS class II drug (Ibuprofen) is enhanced by solventless solid dispersion technique using co-milling. The co-milling is performed in a planetary ball mill using
1:1 wt. ratio of Ibuprofen (drug) and Microcrystalline cellulose, MCC (excipient).The improvement in wettability and dissolution after co-milling are compared with the raw ibuprofen, ball-milled
ibuprofen without any excipient and v-blend mixture of ibuprofen with an excipient. The changes in crystal level properties and reduction in crystallinity due to co-milling are measured using Powder
X-ray diffraction (P-XRD) and Differential Scanning Calorimetry (DSC) respectively. The increased interaction between ibuprofen and MCC as well as hydrogen bond formation is confirmed by Fourier
Transform Infrared Spectroscopy (FTIR). The morphological changes are observed by optical microscopy and Field Emission Scanning Electron Microscopy (FESEM). The miscibility of drug and excipient and
evidence of formation of glassy solutions are demonstrated by Modulated Temperature Differential Scanning Calorimetry (MTDSC) and Raman microscopy. The surface energy and wetting properties are
determined using Inverse Gas Chromatography (IGC) and sessile drop method respectively. The results show that co-milling generates defects, strain, and reduction in crystallite size (changes in
crystal level properties) which are responsible for the improvement of wetting and dissolution (96% in 90 min). Also, with increase in co-milling time, the polar surface energy increases and the
hydrophobic ibuprofen drug surface transforms into hydrophilic surface due to increase in  OH groups of MCC on
the ibuprofen surface. The present work quantified all the above-mentioned parameters including the acidic and basic parameters of co-milled ibuprofen using IGC. The technique improves the wetting
and dissolution of hydrophobic drugs. It can be very well extended to BCS class II and IV drugs in the presence of different hydrophilic excipients.
OH groups of MCC on
the ibuprofen surface. The present work quantified all the above-mentioned parameters including the acidic and basic parameters of co-milled ibuprofen using IGC. The technique improves the wetting
and dissolution of hydrophobic drugs. It can be very well extended to BCS class II and IV drugs in the presence of different hydrophilic excipients.
 OH groups of MCC on
the ibuprofen surface. The present work quantified all the above-mentioned parameters including the acidic and basic parameters of co-milled ibuprofen using IGC. The technique improves the wetting
and dissolution of hydrophobic drugs. It can be very well extended to BCS class II and IV drugs in the presence of different hydrophilic excipients.
OH groups of MCC on
the ibuprofen surface. The present work quantified all the above-mentioned parameters including the acidic and basic parameters of co-milled ibuprofen using IGC. The technique improves the wetting
and dissolution of hydrophobic drugs. It can be very well extended to BCS class II and IV drugs in the presence of different hydrophilic excipients.