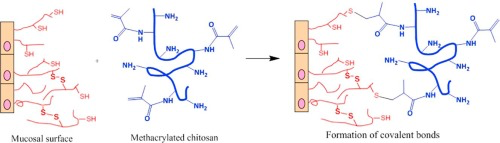
Chitosan is a cationic polysaccharide that exhibits mucoadhesive properties which allow it to adhere to mucosal tissues. In this work, we explored chemical modification of chitosan through its
reaction with methacrylic anhydride to synthesise methacrylated derivative with the aim to improve its mucoadhesive properties. The reaction products were characterised using 1H NMR, FTIR and UV–Vis spectroscopy. 1H NMR and ninhydrin test were used to quantify the degree of methacrylation of chitosan. Turbidimetric analysis of the effect of pH on aqueous
solubility of the polymers revealed that the highly methacrylated derivative remained turbid and its turbidity did not
change from pH 3 to 9. However, solutions of native chitosan and its derivative with low methacrylation remained transparent at pH 6.5 and exhibited a rapid increase in turbidity at pH > 6.5. The
mucoadhesive properties of chitosan and its methacrylated derivatives were evaluated using flow-through method combined with fluorescent microscopy with fluorescein sodium as a model drug. The
retention of these polymers was evaluated on porcine bladder mucosa in vitro. The methacrylated derivatives exhibited greater ability to retain fluorescein sodium on the bladder mucosa compared to
the parent chitosan. Toxicological studies using MTT assay with UMUC3 bladder cells show no significant differences in toxicity between chitosan and its methacrylated derivatives suggesting good
biocompatibility of these novel mucoadhesive polymers.