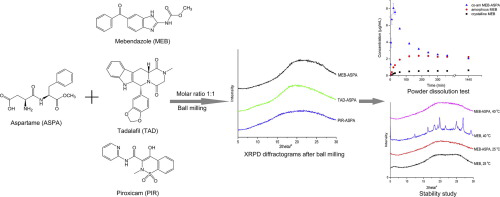
Co-amorphous drug delivery systems are a promising approach to improve the dissolution rate and therefore potentially the oral bioavailability of poorly-water soluble drugs. Several low molecular
weight excipients, for instance amino acids, have previously been shown to stabilize the amorphous form and increase the dissolution rate of drugs. In this study, the feasibility of aspartame, a
methyl ester of the aspartic acid-phenylalanine dipeptide, as a co-former was investigated and compared with the respective single amino acids, both alone and in combination. The poorly water-soluble
compounds mebendazole, tadalafil and piroxicam were chosen as model drugs. In contrast to the single amino acids or the physical mixture of both, all drug-aspartame mixtures became amorphous upon 90
minutes of ball milling. Only a single glass transition temperature (Tg) was detected by modulated differential scanning
calorimetry, which indicates that a homogeneous single-phase co-amorphous system was obtained. Powder dissolution tests showed that the dissolution rates of the drugs from drug-aspartame co-amorphous
samples were increased compared to crystalline drugs. Furthermore, supersaturation was observed for the mebendazole-aspartame and tadalafil-aspartame co-amorphous systems. In conclusion, aspartame
has been shown to be a promising co-former in co-amorphous systems, superior to the single amino acids or their mixtures.