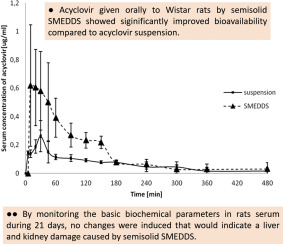
Semisolid self-microemulsifying drug delivery system (SMEDDS) with optimized drugloading capacity, stability, dispersibility in aqueous media and invitro drug release profile, was evaluated in vivo regarding effects on pharmacokinetics of acyclovir, an antiviral with low bioavailability (BA) and short half-life (t1/2). Additional goal of this study was evaluation of safety of this semisolid SMEDDS consisted of medium chain length triglycerides (oil) (10% w/w), macrogolglycerol hydroxystearate (surfactant) (56.25% w/w),polyglyceryl-3-dioleate (cosurfactant) (6.25% w/w), glycerol (cosolvent) (20% w/w), macrogol 8000 (viscosity modifier) (7.5% w/w), and acyclovir (2.5 mg/ml). The study was performed on fully mature white male Wistar rats. The pharmacokinetics of acyclovir was monitored in three groups (1–3) of animals after administration of drug solution (intravenously (IV)), drug suspension (orally) and semisolid SMEDDS (orally),respectively. The determined pharmacokineticparameters were: maximum concentration of acyclovir in serum (Cmax), time taken to reach Cmax (Tmax), areas under time-concentration curves (AUC0–tand AUC0–∞), terminal elimination rateconstant(kel), t1/2, volume ofdistribution (Vd), meanresidence time (MRT), clearance (Cl), zero concentration (C0), steady state volume of distribution (Vss), and BA. Additionally, for safety evaluation, animals were treated orally with aqueous solution of acyclovir, drug-free semisolid SMEDDS and acyclovir-loaded semisolid SMEDDS, during 21 days (groups 4–7). Serum samples of sacrificed animals were used for biochemical analysis of enzymatic activity of alanine transaminase (ALT) and aspartate transaminase (AST), urea, creatinine, and uric acid. Acyclovir administered by semisolid SMEDDS reached twice higher Cmax(0.92 ± 0.21 μg/ml) and has significantly shorter Tmax (14 ± 10.84 min) compared to the suspension of acyclovir (Cmax 0.29 ± 0.09 μg/ml and Tmax 26.00 ± 5.48 min). BA of the drug was significantly increased by semisolid SMEDDS, while the analysis of biochemical parameters excluded damage on function of liver and kidneys caused by the investigated drug delivery system.