Asthma is a chronic respiratory condition characterized by attacks of spasm in the bronchi of the lungs, causing difficulty in breathing. Oral and inhalation routes are generally used for the
treatment of asthma. Terbutaline sulfate (TBS), is a widely used bronchodilator for the treatment of asthma, is available in formulations in the market. However, there is no commercially available
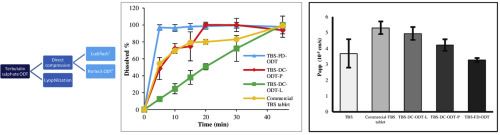
orally disintegrating tablets (ODTs) containing TBS. Therefore, this study was aimed to develop and characterize TBS-containing ODTs. ODTs were prepared using freeze-drying technique and direct
compression using ready-to-use ODT excipients Ludiflash® and Parteck
ODT®. Quality
controls and permeability study across Caco-2 cells were performed. ODTs prepared by direct compression were disintegrated within 3 min, and freeze-dried ODT in 11 s. Acceptance value for content
uniformity was 13.2% for freeze-dried ODTs, and about 22% for direct compressed ODTs. In vitro dissolution test showed that commercial tablet and all ODTs fulfilled the tolerance limit recommended in
TBS conventional tablet monograph of USP. Results of the permeability studies demonstrated that TBS can be classified as a well absorbed compound. All these results indicate that to improve patient
compliance, ODT approach for TBS can be used to improve patient compliance and also for rapid onset of action.
Continue on ScienceDirect