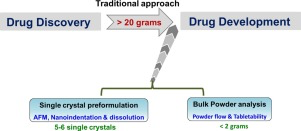
A systematic assessment of key material attributes of active pharmaceutical ingredients (APIs) using minimal material approach is illustrated at the particulate level and bulk level. At a bulk level, flowability improvement of an API through crystal habit manipulation is exemplified. The impact of crystal structures and particulate properties (crystal habit and crystal size) on their mechanical behaviour were addressed by measuring powder tabletability. Bulk powder flow and tabletability were assessed on a small scale using ring shear tester and using a single punch compaction simulator, which can be easily integrated during the process of solid form screening activity for early preformulation studies. In addition, mechanical responses on single crystals were evaluated with nanoindentation. Indentation performed on three faces of piroxicam single crystals resulted reproducible results when compared with the literature, indicating the efficiency of this technique to perform single crystal characterization. The study highlights that, the early assessment of key material properties during solid form screening can aid in selecting optimal solid forms provided physicochemical properties are acceptable.