Three-dimensional printing (3DP), though developed for nonmedical applications and once regarded as futuristic only, has recently been deployed for the fabrication of pharmaceutical products.
However, the existing feeding materials (inks and filaments) that are used for printing drug products have various shortcomings, including the lack of biocompatibility, inadequate extrudability and
printability, poor drug loading, and instability. Here, we have sought to develop a filament using a single pharmaceutical polymer, with no additives, which can be multi-purposed and manipulated by
computational design for the preparation of tablets with desired release and absorption patterns. As such, we have used hydroxypropyl-methylcellulose (HPMC) and diltiazem, a model drug, to prepare
both drug-free and drug-impregnated filaments, and investigated their thermal and crystalline properties, studied the cytotoxicity of the filaments, designed and printed tablets with various infill
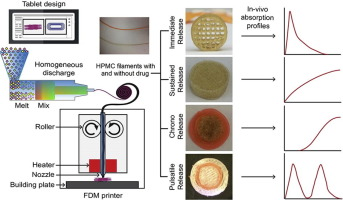
densities and patterns. By alternating the drug-free and drug-impregnated filaments, we fabricated various types of tablets, studied the drug release profiles, and assessed oral absorption in rats.
Both diltiazem and HPMC were stable at extrusion and printing temperatures, and the drug loading was 10% (w/w). The infill density, as well as infill patterns, influenced the drug release profile,
and thus, when the infill density was increased to 100%, the percentage of drug released dramatically declined. Tablets with alternating drug-free and drug-loaded layers showed delayed and
intermittent drug release, depending on when the drug-loaded layers encountered the dissolution media. Importantly, the oral absorption patterns accurately reproduced the drug release profiles and
showed immediate, extended, delayed and episodic absorption of the drug from the rat gastrointestinal tract (GIT). Overall, we have demonstrated here that filaments for 3D printers can be prepared
from a pharmaceutical polymer with no additives, and the novel computational design allows for fabricating tablets with the capability of producing distinct absorption patterns after oral
administration.