The maximum achievable concentration of a drug in solution is dictated by the chemical potential of the solid form. Because an amorphous solid has a higher chemical potential than the corresponding
crystal form, in the absence of phase transformations, a higher transient solubility is expected. However, the chemical potential of an amorphous drug can be reduced by mixing with another component.
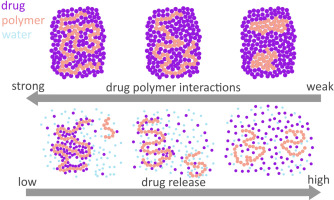
Therefore, upon mixing with a polymer to form an amorphous solid dispersion (ASD), the maximum solution concentration achieved can be potentially altered, in particular if the polymer is poorly
soluble in the dissolution medium. Such changes in the chemical potential of the drug may be a critical factor in determining the maximum achievable solution concentration, and could alter the
crystallization driving force of the drug. Therefore, the aim of this study was to gain insights into the impact of poorly soluble polymers on the “amorphous solubility” of drugs formulated as
amorphous solid dispersions. Lopinavir was selected as a model drug with a low crystallization tendency, enabling determination of the amorphous solubility as a function of ASD composition. Model
polymers included cellulose acetate (CA), CA phthalate (CAP), ethylcellulose (EC), Eudragit® RL PO (EUD), hydroxypropylmethylcellulose (HPMC), HPMC acetate succinate (HPMCAS), and HPMC phthalate
(HPMCP). The “amorphous solubility” of the drug alone was determined and then the changes in maximum achievable concentration were measured as a function of drug loading. Drug-polymer interactions
were characterized using infrared spectroscopy (IR), differential scanning calorimetry (DSC) and moisture sorption analysis. The results showed that the maximum achievable concentration (“amorphous
solubility”) of lopinavir varied with the extent of drug-polymer interactions, as well as the drug weight fraction in the ASD. This information is of great value when evaluating the maximum
achievable concentration of amorphous systems formulated with pH responsive polymers, and should contribute to a broader understanding of drug phase behavior in the context of ASDs.