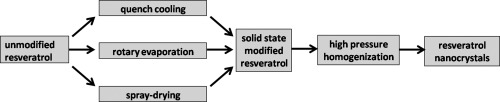
Production of nanosuspension for drug bioavailability improvement has been accepted and frequently used in pharmaceutical industry. Normally, the preparation started from crystalline drug. However,
some previous studies found that amorphous drug powder obtained from processes such as spray-drying might contribute to nanosizing process, i.e. reduced homogenization cycles and smaller achievable
particle size. The mechanism behind this improved behavior was still unclear. In order to systematically investigate, three technologies, i.e. spray-drying, rotary evaporation and quench cooling,
were used to modify drug powders, which were all processed by high pressure homogenization (HPH). Predominately amorphous solids have been obtained after processing both spray-drying (spherical
particle) and rotary evaporation (no obvious morphology modification). However, those samples could not be beneficial to efficiency of nanosizing process. The smallest particle size of
nanosuspensions was achieved by using spray-dried solid possessing predominately crystallinity (slightly reduced crystallinity) and changed morphology (more breakage points). Therefore, both the
modification of morphology and solid state control were confirmed crucial for the followed HPH efficiency. In another part of this study, it was found that the directly dried nanosuspensions used in
many previous studies were actually not suitable for a proper solid state analysis by differential scanning calorimetry (DSC). A separation step of drug and stabilizer/surfactant is strongly
recommended in order to improve the validity of solid state measurement for drug nanosuspensions.