The objective of this study was to assess the efficacy and the capability of a novel ethylcellulose-based dry-coating system to obtain prolonged and stable release profiles of caffeine-loaded pellets. Lauric and oleic acids at a suitable proportion were used to plasticize ethylcellulose. The effect of coating level, percentage of drug loading, inert core particle size, and composition of the coating formulation including the anti-sticking agent on the drug release profile were fully investigated. A coating level of 15% w/w was the maximum layered amount which could modify the drug release. The best controlled drug release was obtained by atomizing talc (2.5% w/w) together with the solid plasticizer during the dry powder-coating process. SEM pictures revealed a substantial drug re-crystallization on the pellet surface, and the release studies evidenced that caffeine diffused through the plasticized polymer acting as pore former. Therefore, the phenomenon of caffeine migration across the coating layer had a strong influence on the permeability of the coating membrane. Comparing dry powder-coated pellets to aqueous film-coated ones, drug migration happened during storage, though more sustained release profiles were obtained. The developed dry powder-coating process enabled the production of stable caffeine sustained release pellets. Surprisingly, the release properties of the dry-coated pellets were mainly influenced by the way of addition of talc into the dry powder-coating blend and by the drug nature and affinity to the coating components. It would be interesting to study the efficacy of novel coating system using a different API.
Conclusion
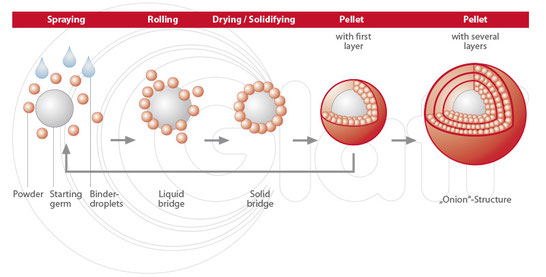
An innovative dry powder-coating technology was used to apply a functional ethylcellulose-based coating upon CAF- loaded pellets, avoiding the use of organic solvents or water. The caffeine release could be modulated varying different formulation parameters: coating level and size of sugar spheres highly influenced the dissolution profiles, while negligible changes were observed varying the drug loading and the amount of plasticizers. Glidants were essential to reduce pellet aggregation but it was found that they could not be added in blend with ethylcellulose, as usually reported, since talc interfered with the powder adhesion upon sugar spheres and consequently with the film formation. The best drug release performance was achieved by atomizing talc 2.5% w/w with the main solid plasticizer, lauric acid.
Finally, this research revealed that, in addition to the effect of coating level and of the way of addition of the glidant, the release properties of coated pellets were influenced by the nature of the drug and its affinity with the coating components. In this study, caffeine demon- strated the ability to migrate through the ethylcellulose film layer. This phenomenon was favored by the coating process as well as the curing step and influenced the properties of the coated pellets. Hence, the caffeine dissolution behavior was affected by the drug re-crystallization on the pellet surface. Concerning the stability issue, the formulations showed that drug migration did not occur during storage. Comparing dry powder-coated pellets with film-coated ones, the phenomenon of CAF re-crystallization on the pellets surface occurred in both samples albeit to a different extent. Moreover, drug migration from film-coated pellets hap- pened during aging. Replacing caffeine with another active pharmaceutical ingredient of the same BCS Class, the investigated ethylcellulose-based coating system could be advantageously exploited.