Abstract
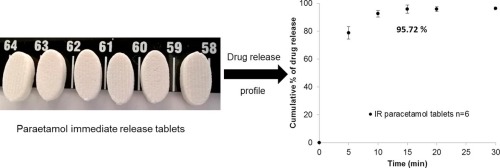
The manufacture of immediate release high drug loading paracetamol oral tablets was achieved using an extrusion based 3D printer from a premixed water based paste formulation. The 3D printed tablets demonstrate that a very high drug (paracetamol) loading formulation (80% w/w) can be printed as an acceptable tablet using a method suitable for personalisation and distributed manufacture. Paracetamol is an example of a drug whose physical form can present challenges to traditional powder compression tableting. Printing avoids these issues and facilitates the relatively high drug loading. The 3D printed tablets were evaluated for physical and mechanical properties including weight variation, friability, breaking force, disintegration time, and dimensions and were within acceptable range as defined by the international standards stated in the United States Pharmacopoeia (USP). X-ray Powder Diffraction (XRPD) was used to identify the physical form of the active. Additionally, XRPD, Attenuated Total Reflectance Fourier Transform Infrared spectroscopy (ATR-FTIR) and differential scanning calorimetry (DSC) were used to assess possible drug-excipient interactions. The 3D printed tablets were evaluated for drug release using a USP dissolution testing type I apparatus. The tablets showed a profile characteristic of the immediate release profile as intended based upon the active/excipient ratio used with disintegration in less than 60 s and release of most of the drug within 5 min. The results demonstrate the capability of 3D extrusion based printing to produce acceptable high-drug loading tablets from approved materials that comply with current USP standards.
Conclusions
Extrusion based 3D printing of paracetamol immediate release tablets with a very high drug loading (80 % w/w) was successfully demonstrated. The 3D printed tablets released more than 90 % of the active within 10 min. XRPD, FTIR, DSC and SEM data show that the paracetamol form was unaffected by the printing and that there was no detectable interactions between the paracetamol and the chosen excipients (PVP K25 and CCS). The 3D printed paracetamol tablets were also evaluated for weight variation, hardness, friability, disintegration time, and size and dimension and were within acceptable range as defined by the international standards stated in the USP. The present work is a step towards the practical demonstration and validation of 3D printing of tablets with high drug loading for the tailored manufacture of medicines and personalized care and treatment. The work clearly demonstrates the capability of 3D extrusion based printing to produce acceptable tablets from approved materials that comply with current USP standards and that if a suitable regulatory and quality environment can be established that this could be achieved in a distributed manufacture model.
Recommended for you