Both pharmacological and economic factors drive the decision to use these technologies.
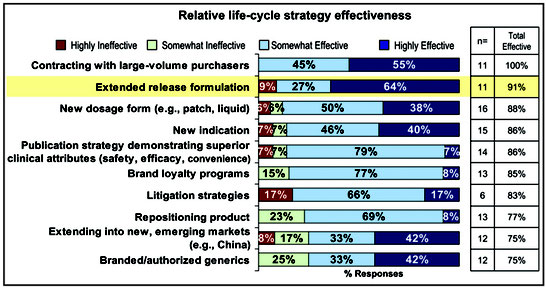
This article by Peggy Wright published in Pharmaceutical Commerce in 2014 describes the potential of extended release formulations in life cycle management of drug products. Has the situation changed in the meantime?
The FDA clarified the regulatory thicket surrounding the 505(b)(2) approval pathway for drug formulations just over a decade ago. In the intervening years, this pathway has become an increasingly popular way for drug makers to commercialize products. The 505(b)(2) pathway allows companies to make modest changes to an already approved drug and get continued market exclusivity for from three to as many as seven years. And among those changes, one of the more popular is to develop a controlled-release, long-acting, extended-release, or XR version of the drug, all of which constitute a modified-release approach.
Some drugs, from their first launch, are formulated to provide a relatively steady dose to the patient over an extended period of time, either to provide more effective therapy or better patient acceptance. Transdermal patches or medical devices like infusion pumps can serve a similar purpose. Most often XR formulations show up because a company is attempting to extend the exclusivity of its proprietary product, a form of life-cycle management for the drug as it nears patent expiration (expiry).
Recommended for you
- Developing a quality by design approach to model tablet dissolution testing: an industrial case study
- Film Coating Excellence by Biogrund
- Excipient DMF 3rd Quarter 2017
Don't miss any blog article - Sign up for the weekly blog digest!