Abstract
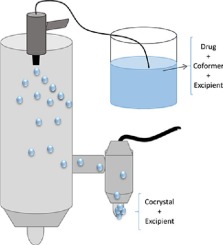
Spray drying is a well-established scale-up technique for the production of cocrystals. However, to the best of our knowledge, the effect of introducing a third component into the feed solution during the spray drying process has never been investigated. Cocrystal formation in the presence of a third component by a one-step spray drying process has the potential to reduce the number of unit operations which are required to produce a final pharmaceutical product (e.g. by eliminating blending with excipient). Sulfadimidine (SDM), a poorly water soluble active pharmaceutical ingredient (API), and 4-aminosalicylic acid (4ASA), a hydrophilic molecule, were used as model drug and coformer respectively to form cocrystals by spray drying in the presence of a third component (excipient). The solubility of the cocrystal in the excipient was measured using a thermal analysis approach. Trends in measured solubility were in agreement with those determined by calculated Hansen Solubility Parameter (HSP) values. The ratio of cocrystal components to excipient was altered and cocrystal formation at different weight ratios was assessed. Cocrystal integrity was preserved when the cocrystal components were immiscible with the excipient, based on the difference in Hansen Solubility Parameters (HSP). For immiscible systems (difference in HSP >9.6 MPa0.5), cocrystal formation occurred even when the proportion of excipient was high (90% w/w). When the excipient was partly miscible with the cocrystal components, cocrystal formation was observed post spray drying, but crystalline API and coformer were also recovered in the processed powder. An amorphous dispersion was formed when the excipient was miscible with the cocrystal components even when the proportion of excipient used as low (10% w/w excipient). For selected spray dried cocrystal-excipient systems an improvement in tableting characteristics was observed, relative to equivalent physical mixtures.
Conclusions
This work demonstrates that the introduction of a third component into the feed solution/suspension prior to spray drying can result in a cocrystal embedded in excipient matrix. Cocrystal formation can also occur when more than one excipient is added to the spray drying feed solution/suspension. The difference in HSP between the cocrystal components and the excipient can be used as a general parameter to predict if cocrystal formation will occur. However, as was seen when the cocrystal components were co-spray dried with chitosan, other factors such as the acidic/basic nature of the excipient can influence whether cocrystal formation can occur. The difference in HSP can also be used to predict the ratio at which a cocrystal can form when co-spray dried with an excipient. Co- spray drying an excipient with the cocrystal components can result in cocrystal formation, regardless of the crystalline or amorphous nature of the excipient. As spray drying is a scalable unit operation used in the pharmaceutical industry, co-spray drying with an excipient can reduce the number of unit operations required to produce a final pharmaceutical product, as a separate blending step of the cocrystal and excipient could be avoided.