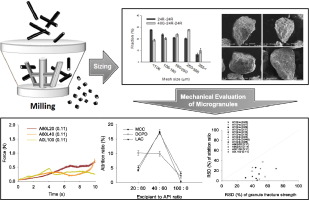
Abstract
This study focuses on understanding the physicochemical principles for the preparation of high drug-loaded microgranules with a desired size distribution and mechanical properties. Mesalamine was selected as a model drug, and microgranulation was performed using an extruder and a conical screen mill. Throughout the processes, factors including the binder to powder ratio, the shape of the impeller, and type and hole size of the screen significantly affected the size distribution of the microgranules. In particular, the number of milling stages made a noticeable difference, as the addition of a pre-milling stage could increase the yield of microgranules within the target interval by facilitating the particle escape from the screen. Moreover, the effect of three commonly used excipients, microcrystalline cellulose, dibasic calcium phosphate dihydrate, and lactose monohydrate, were investigated with the expectation of enhancing the mechanical properties of microgranules. Unexpectedly, however, the addition of excipients resulted in a rather negative effect on the friability of microgranules owing to the inhomogeneous distribution of the binder polymer. An attrition test was determined to be a suitable method for evaluating the mechanical properties of microgranules due to its dynamic test condition. In contrast, tests performed using a texture analyzer were static, which in turn distorted the strength of microgranules. As various active pharmaceutical ingredients and excipients have their own characteristics, investigation into their physicochemical fundamentals is an essential prerequisite for the successful preparation of a high drug-loaded microparticle system.
Conclusions
In this study, the overall processes for the preparation of high drug-loaded microgranules using an extruder and conical screen mill were investigated. Together, three commonly used excipients were added and evaluated for the improvement in mechanical properties of the microgranules. Factors including shape of the impeller, type of screen, and binder to powder ratio significantly affected the size distribution of the microgranules. Particularly, multi-stage milling was found to enhance the yield of target size particles due to the elevated screen-escape efficiency. The type and grade of the binder polymer were also closely related to the properties of microgranules. When excipients were added to the API, mechanical natures of the microgranules were modified to plastic or brittle due to the innate characteristics of the excipients. Static tests using a texture analyzer had some limitations in that they could not measure the strength of the microgranules with plastic deformation, whereas attrition tests were suitable for comparing the mechanical properties of various microgranules with high reproducibility. In addition, single granule fracture tests had a possibility of distortion when interpreting the results. Added excipients resulted in increased fragility owing to the different absorption ability of each composition, which brought about an uneven distribution of the binder polymer. In summary, to prepare high drug-loaded microgranules within a desired size range and with appropriate mechanical properties, first the sizing mechanism and characterization of the physicochemical properties of each component must be studied.