Abstract
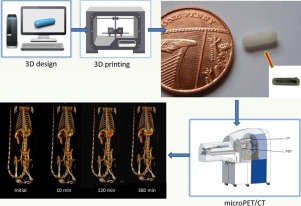
Fused deposition modelling (FDM) 3D printing (3DP) is a revolutionary technology with the potential to transform drug product design in both the pre-clinical and clinical arena. The objective of this pilot study was to explore the intestinal behaviour of four different polymer-based devices fabricated using FDM 3DP technology in rats. Small capsular devices of 8.6mm in length and 2.65mm in diameter were printed from polyvinyl alcohol-polyethylene glycol graft-copolymer (PVA-PEG copolymer, Kollicoat IR), hydroxypropylcellulose (HPC, Klucel), ethylcellulose (EC, Aqualon N7) and hypromellose acetate succinate (HPMCAS, Aquasolve-LG). A smaller sized device, 3.2mm in length and 2.65mm in diameter, was also prepared with HPMCAS to evaluate the cut off size of gastric emptying of solid formulations in rats. The devices were radiolabelled with Fluorodeoxyglucose (18F-FDG) and small animal positron emission tomography/computed tomography (microPET/CT) was used to track the movement and disintegration of the fabricated devices in the rats. The PVA-PEG copolymer and HPC devices disintegrated after 60min following oral administration. The EC structures did not disintegrate in the gastrointestinal tracts of the rats, whereas the HPMCAS-based systems disintegrated after 420min. Interestingly, it was noted that the devices which remained intact over the course of the study had not emptied from the stomach of the rats. This was also the case with the smaller sized device. In summary, we report for the first time, the use of a microPET/CT imaging technique to evaluate the in vivo behaviour of 3D printed formulations. The manipulation of the 3D printed device design could be used to fabricate dosage forms of varying sizes and geometries with better gastric emptying characteristics suitable for rodent administration. The increased understanding of the capabilities of 3DP in dosage form design could, henceforth, accelerate pre-clinical testing of new drug candidates in animal models.
Conclusions
This is the first study to demonstrate the viability of FDM 3DP as a meaningful and feasible method of manufacturing of small capsular devices, as evaluated in vivo studies with the utilisation of microPET/CT imaging. The devices were successfully 3D printed using four different polymer filaments: Kollicoat IR (immediate release), Klucel EF (water soluble), Aqualon N7 (insoluble polymer) and Aquasolve-LG (pH dependent). MicroPET/CT imaging offered valuable in vivo data of the behaviour of 3DP devices, using the rat as a model animal. Kollicoat IR and Klucel EF devices disintegrated within 1 to 2h, whilst Aquasolve-LG devices took longer than 7h to disintegrate. Aqualon N7 devices did not disintegrate or release the tracer and gastric emptying was not observed for any of the devices. In addition, this study contributed to the identified inconsistencies regarding the lack of gastric emptying of modified release in in vivo studies. Therefore, this pilot study confirmed that 3DP is capable of printing solid dosage forms, however, further work should be employed to fabricate different geometries and smaller devices to successfully empty from the stomach of rats. In summary, the work has highlighted the potential of FDM 3DP in the design of formulations suitable for pre-clinical testing of drugs, and thus, offer advantages towards targeted release and personalised dosing.