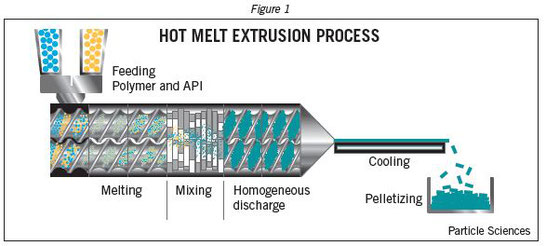
Amorphous solid dispersions (SDs) are considered as one of the most effective strategies for the formulation of poorly water-soluble compounds. The active compound is dispersed in an inert carrier composed of a polymer and active excipients. Since the drug is amorphous, there is typically an increase in apparent solubility as well as dissolution rate. Various methods are employed for manufacturing of SDs, nevertheless, hot-melt extrusion (HME) has become one of the most common process techniques. Indeed, as a solvent-free, one-step continuous process allowing the production of a wide variety of solid dosage forms, HME has emerged as an attractive method. Among the excipients that can be used for SD development, lipid-based excipients are particularly interesting for the formulation of lipophilic compounds. They act as drug solubilizers and stabilizers by improving the chemical and physical stability of drugs. Among poorly water-soluble compounds those exhibiting both high crystallinity and lipophilicity are particularly challenging and require specific formulation considerations. A simple polymeric system might not be sufficient to obtain amorphous SDs. This can lead to sophisticated systems in structure and composition, which are hence rather complex to characterize by means of conventional analysis methods.
The present thesis consists of four studies that aim at developing novel lipid-based formulations for crystalline lipophilic compounds by means of HME and that introduce new characterization methods. For this purpose, β-carotene (BC) was selected as a high melting point, poorly water- soluble model compound.
The objective of the first study was to compare the ability of state-of-the-art methods to detect the presence of low-dose crystalline compounds in lipid matrices. Sensitivity issues were encountered using conventional methods, therefore a new analytical tool was introduced. The novel flow-through cross-polarized imaging combined the advantages of analyzing large sample sizes and the high sensitivity of a microscopic technique. Small amounts of crystalline materials could easily be detected and an upper limit of the kinetic solubility of the model compound could be estimated.
http://edoc.unibas.ch/56002/1/Thesis_Camille%20Adler%20Electronic%20version.pdf
Recommended for you
- Accelerating pharmaceutical development through predictive stability approaches
- AFFINISOL™ HPMC HME for Hot Melt Extrusion
- Excipient DMF 3rd Quarter 2017