Oral vaccines present an attractive alternative to injectable vaccines for enteric diseases due to ease of delivery and the induction of intestinal immunity at the site of infection. However,
susceptibility to gastrointestinal proteolysis, limited transepithelial uptake and a lack of clinically acceptable adjuvants present significant challenges. A further challenge to mass vaccination in
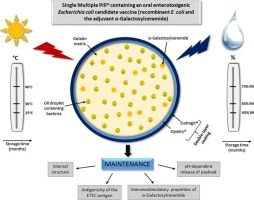
developing countries is the very expensive requirement to maintain the cold chain. We recently described the effectiveness of a Single Multiple Pill® (SmPill®) adjuvanted capsule approach to enhance the
effectiveness of a candidate enterotoxigenic Escherichia coli (ETEC) oral vaccine. Here it was demonstrated that this delivery system maintains the antigenicity of ETEC colonisation factor
antigen I (CFA/I) and the immunostimulatory activity of the orally active α-Galactosylceramide (α-GalCer) adjuvant after storage of SmPill® minispheres under room temperature and extreme storage
conditions for several months. In addition, the internal structure of the cores of SmPill®minispheres and antigen release features at intestinal pH were found to be preserved under all these
conditions. However, changes in the surface morphology of SmPill® minispheres leading to the antigen release at gastric pH were observed after a few weeks of storage under extreme conditions.
Those modifications were prevented by the introduction of an Opadry® White film coating layer between the core of SmPill® minispheres and the enteric coating. Under these conditions,
protection against antigen release at gastric pH was maintained even under high temperature and humidity conditions. These results support the potential of the SmPill® minisphere approach
to maintain the stability of an adjuvanted whole cell killed oral vaccine formulation.
Recommended for you
- Reformulation of Tablets to resolve sticking & picking issues
- Captisol - modified cyclodextin for improved solubility, stability, bioavailability
- Excipient DMF List