- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
04. October 2018
Praziquantel is an antiparasitic drug used for decades. Currently, the praziquantel commercial preparation is a racemic mixture, inwhich only the levo-enantiomer possesses anthelmintic activity. The knowledge of its properties in the solid state and other chemical-physical properties is necessary for improving its efficacy andapplications. Drug solid dispersions were prepared with calcium carbonate at 1:5 drug to excipient weight ratio by solventevaporation method. Then, the modification of the...
17. July 2018
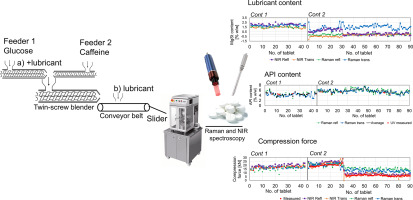
By the advent of continuous pharmaceutical manufacturing, fast and accurate characterization of product quality has become of a major interest. Although it also promotes the real-time release testing approach, so far mainly content uniformity studies were performed by near-infrared (NIR) spectroscopy. This paper proposes the simultaneous application of NIR and Raman spectroscopy to nondestructively analyze the critical quality attributes of continuously produced tablets in a real-time release...
07. July 2018
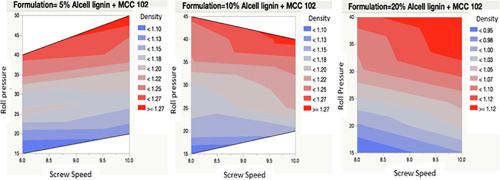
In this study, a process map was developed in an effort to improve the understanding of dry granulation of pharmaceutical excipients by roll compaction process, and to implement the quality-by-design (QbD) approach. Through development of the process map, a correlation was made between the critical process parameters (roll pressure, screw speed), and critical quality attributes (density of ribbons and granule size). This method reduces development time, quantity of materials required and cost....
15. October 2017
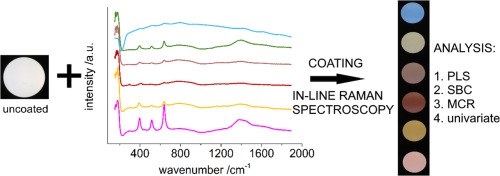
Endpoints of coating processes for colored tablets were determined using in-line Raman spectroscopy. Coatings were performed with six commercially available formulations of pink, yellow, red, beige, green and blue color. The coatings were comprising pigments and/or dyes, some causing fluorescence and interfering the Raman signal. Using non-contact optics, a Raman probe was used as process analytical technology (PAT) tool, and acquired spectra were correlated to the sprayed mass of aqueous...
14. October 2017
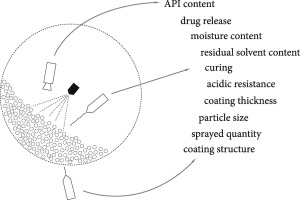
Over the last two decades, regulatory agencies have demanded better understanding of pharmaceutical products and processes by implementing new technological approaches, such as process analytical technology (PAT). Process analysers present a key PAT tool, which enables effective process monitoring, and thus improved process control of medicinal product manufacturing. Process analysers applicable in pharmaceutical coating unit operations are comprehensibly described in the present article. The...
24. March 2017
Abstract Recent work established polymer strip films as a robust platform for delivery of poorly water-soluble drug particles. However, a simple means of manipulating rate of drug release from films with minimal impact on film mechanical properties has yet to be demonstrated. This study explores the impact of film-forming polymer molecular weight (MW) and concentration on properties of polymer films loaded with poorly water-soluble drug nanoparticles. Nanoparticles of griseofulvin, a model...
22. March 2017
Abstract The purpose of this study is to characterize controlled release matrix tablets of captopril and to find out the physicochemical properties that have an effect on the mucoadhesion process. The hydrophilic matrix tablets contain captopril, microcrystalline cellulose, barium sulfate, ascorbic acid, ethylcellulose N100, hydroxypropylmethylcellulose K15M, talc, magnesium stearate and colloidal silicon dioxide. The physicochemical properties of the formulations have been characterized using...
12. March 2017
Abstract Novel excipients are entering the market to enhance the bioavailability of drug particles by having a high porosity and thus providing a rapid liquid uptake and disintegration to accelerate subsequent drug dissolution. One example of such a novel excipient is functionalised calcium carbonate (FCC), which enables the manufacture of compacts with a bimodal pore size distribution consisting of larger inter-particle and fine intra-particle pores. Five sets of FCC tablets with a target...
20. February 2017
Abstract Near-infrared chemical imaging (NIR-CI) with high-speed cameras based on the push-broom acquisition principle is a rapidly-evolving and can be used for a variety of purposes, from classification (and sorting) of products to mapping spatial distribution of materials. The present study examined if NIR-CI is suitable for tablet manufacturing. To that end, the tablets were introduced into the CI system via a flat belt conveyor. A formulation, which consisted of 4wt.%–6wt.% caffeine,...
11. January 2017
Abstract With the implementation of quality by design (QbD), critical attributes of raw material (drug substance and excipients) are of significantly importance in pharmaceutical manufacturing process. It is desirable for the quality control of critical material attributes (CMAs) of excipients to ensure the quality of end product. This paper explored the feasibility of an at-line method for the quantitative analysis of hydroxypropoxy group in hydroxypropyl methylcellulose (HPMC) with near...