- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
21. September 2018
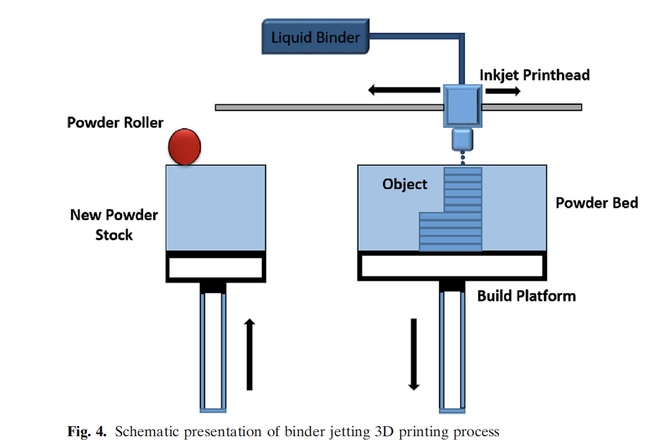
Three-dimensional (3D) printing was discovered in the 1980s, and many industries have embraced it, but the pharmaceutical industry is slow or reluctant to adopt it. Spiritam® is the first and only 3D-printed drug product approved by FDA in 2015. Since then, the FDA has not approved any 3D-printed drug product due to technical and regulatory issues. The 3D printing process cannot compete with well-established and understood conventional processes for making solid dosage forms. However,...
11. September 2018
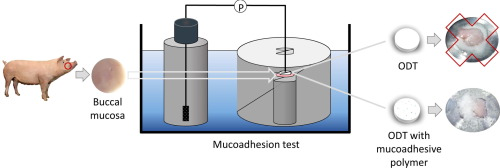
Oromucosal drug delivery is necessary when a local effect in the oral cavity is required. Bioadhesive formulations should be advantageous because a larger fraction of the active principal is retained at the site of action allowing for an enhanced and prolonged effect. Despite a variety of mucoadhesion test systems being described in literature, none of these in-vitro tests does relate to physiological conditions in the oral cavity and suites for the testing of complete dosage forms, e.g....
05. September 2018
This study aimed to enhance the dissolution rate of isradipine and to avoid the problem of first-pass metabolism using oral fast dissolving films (OFDFs). Isradipine was complexed with hydroxypropyl-beta-cyclodextrin (HP-β-CD) using the solvent co-evaporation technique to produce an isradipine inclusion complex with increased solubility. This complex was evaluated for its solubility and dissolution behavior compared with the free drug. Then, the inclusion complex was formulated in OFDFs. The...
29. August 2018
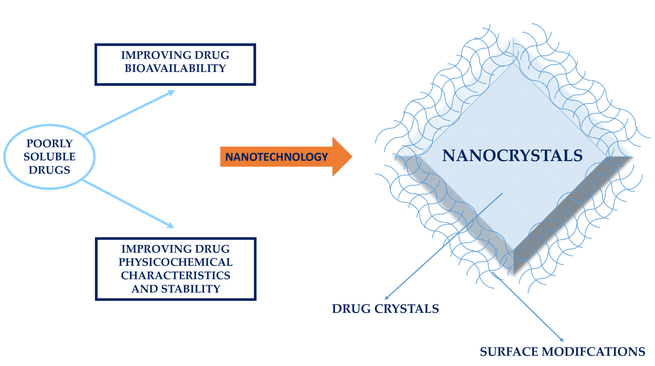
Many approaches have been developed over time to overcome the bioavailability limitations of poorly soluble drugs. With the advances in nanotechnology in recent decades, science and industry have been approaching this issue through the formulation of drugs as nanocrystals, which consist of “pure drugs and a minimum of surface active agents required for stabilization”. They are defined as “carrier-free submicron colloidal drug delivery systems with a mean particle size in the nanometer...
10. July 2018
Future Market Insights (FMI) delivers key insights on the global biologic excipients market in its upcoming report titled “Biologic Excipients Market: Global Industry Analysis 2013–2017 and Opportunity Assessment 2018 – 2028”. In terms of revenue, the global biologic excipients market is projected to register a CAGR of 3.6% over the forecast period owing to various factors, regarding which FMI offers detailed insights and forecasts in this report. Global Biologic Excipients Market:...
10. November 2017
The design and development of nanoparticle- and microparticle-based delivery systems for the encapsulation, protection, and controlled release of active agents has grown considerably in the agrochemical, cosmetic, food, personal care, and pharmaceutical industries.
26. October 2017
Mycotoxins are secondary toxic metabolites that are produced by fungi representing threats to human and animal health. The objective of this study was to evaluate the adsorption capacity of Chitosan (CHI), and three cellulosic polymers (HPMC, CMC, and MCC), on six mycotoxins (AFB1; FUB1; OTA; T-2; DON; and, ZEA) using an in vitro digestive model for poultry. The adsorbent capacity of the materials in the supernatant of each compartment was evaluated by a non-competitive chemiluminescent assay....
16. September 2017
Results of experiments on prolonged release of paracetamol from polymer matrix tablets with cross-linked poly(acrylic acid) Carbopol®Ultrez 21 as the prolonging polymer were reported. The effects of factors such as tableting technology, type of alkaline agent, and ratio of Carbopol® to alkaline agent on paracetamol release kinetics were studied. It was established that matrices prepared by wet granulation had the best potential for prolonging the release. The ratio of Carbopol®to alkaline...
25. March 2017
Abstract The study discusses the synthesis of polymer-silica composites comprising water soluble drug (ibuprofen sodium, IBS). The polymers selected for this study were poly(TRIM) and poly(HEMA-co-TRIM) produced in the form of permanently porous beads via the suspension-emulsion polymerization method. The acid and base set ternary composites were prepared by the saturation of the solid dispersions of drug (poly(TRIM)-IBS and/or poly(HEMA-co-TRIM)-IBS) with TEOS, and followed by their exposition...
24. March 2017
Abstract Recent work established polymer strip films as a robust platform for delivery of poorly water-soluble drug particles. However, a simple means of manipulating rate of drug release from films with minimal impact on film mechanical properties has yet to be demonstrated. This study explores the impact of film-forming polymer molecular weight (MW) and concentration on properties of polymer films loaded with poorly water-soluble drug nanoparticles. Nanoparticles of griseofulvin, a model...