- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
19. August 2018
The staff of the FDA’s Center for Drug Evaluation and Research (CDER) always tries to utilize cutting-edge science and up-to-date process management, befitting our stature as the global “gold standard” in drug regulation. Maintaining that standard requires us to keep up with evolving technology and the latest scientific, medical and regulatory advances. Current factors impacting drug development include the genomic revolution, the rise of targeted therapy, the availability of digital...
20. February 2017
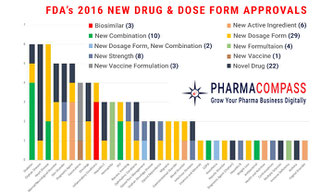
After 2 years of sky-high approval numbers, the US Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research approved 22 novel drugs in 2016, down from the 19-year high of 45 in 2015. The FDA also approved many new dose forms, formulations, combination products and vaccines. This week, PharmaCompass, shares its analysis of the new drug approvals by the FDA in 2016. Reasons behind the low approvals in 2016 Of the 22 novel drugs approved by the FDA, the FDA approved 9...
08. November 2016
Abstract In the current study, we investigated the metoprolol absorption kinetics of an in-house produced oral sustained-release formulation, matrices manufactured via prilling, and two commercially available formulations, ZOK-ZID® (reservoir) and Slow-Lopresor® (matrix) in both New Zealand White rabbits and Beagle dogs, using a population pharmacokinetic analysis approach. The aim of this study was to compare the in vivo pharmacokinetic (PK) profiles of different formulations based on...
03. February 2016
Objective - Efficacy and safety of a novel multiple-unit hydromorphone once daily (HOD) was compared to an established hydromorphone twice daily (HTD) regimen in patients with moderate-to-severe chronic pain. Design and methods - The results from a randomized, double-blind, multicenter, cross-over trial in patients (n = 37) with chronic malignant or non-malignant pain are reported. Primary efficacy parameter was current pain on 0-100 mm VAS assessed 4-times daily and prior to intake of rescue...
30. January 2016
This study was aimed at isolating, characterizing and evaluating the excipient functionalities of a polymer from watermelon seeds. After oil extraction, the marc was dispersed in water, agitated, filtered, the polymer harvested as the sediment in the filtrate and purified. The polymer was characterized; used as excipient in tablet formulation; and tablets' qualities were assessed using standard protocols. Physicochemical tests revealed polymer as non-polysaccharide, amorphous biomaterial with...
25. December 2014
http://www.thepharmatimes.in/index.php/extras/jstuff/content-views/category-list-view/1341-dow-colorcon-alliance-launches-a-new-excipient-for-the-indian-subcontinent