- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
09. November 2017
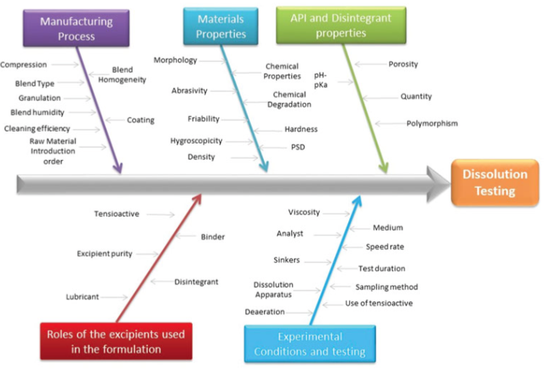
This study applied the concept of Quality by Design (QbD) to tablet dissolution. Its goal was to propose a quality control strategy to model dissolution testing of solid oral dose products according to International Conference on Harmonization guidelines. The methodology involved the following three steps.
12. September 2017
Abstract There has been significant growth in the use of modeling tools to accelerate development and enhance pharmaceutical quality. Among these are empirical and semi-empirical modeling of accelerated stability studies which can be used to predict product shelf-life (Waterman, Pharm Res 24:780–790, 2007; (Wu et al., AAPS Pharm Sci Tech,16:986–991, 2016); (Lavrich, Rapid Development of Robust Stability Models Using Semi-Empirical Design Space, AAPS Webinar, 2016)). These approaches coupled...