- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
13. February 2018
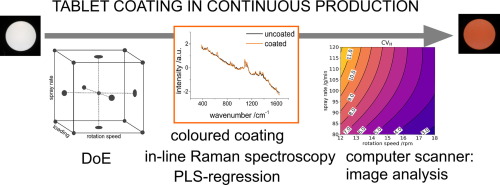
The aim of the study was to optimize a tablet coating process for a continuous manufacturing line. High throughputs should be achieved while inter-tablet coating variability should be as small as possible. Drug-free cores were coated with a colored suspension. All processes were monitored in-line with Raman spectroscopy. A statistical design of experiment was performed to find optimum process parameters.
09. November 2017
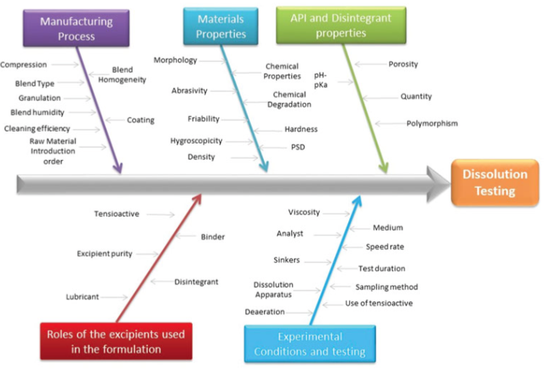
This study applied the concept of Quality by Design (QbD) to tablet dissolution. Its goal was to propose a quality control strategy to model dissolution testing of solid oral dose products according to International Conference on Harmonization guidelines. The methodology involved the following three steps.
23. October 2017
The development of novel excipients with enhanced functionality has been explored using particle engineering by co-processing. The aim of this study was to improve the functionality of tapioca starch (TS) for direct compression by co-processing with gelatin (GEL) and colloidal silicon dioxide (CSD) in optimized proportions.
07. August 2017
The process, equipment, and technology for developing and manufacturing oral solid-dosage forms are well defined. Nonetheless, designing a drug product formulation that achieves the desired properties of the target profile both in magnitude and robustness is a multi-dimensional, and generally, constrained optimization problem, observes Aaron Goodwin, principal investigator, Research and Development, Capsugel.