- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
07. September 2018
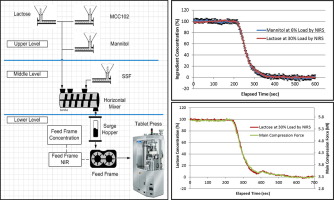
The material residence time distribution in a continuous manufacturing process can be utilized to develop, design and justify the process control strategy. This paper successfully demonstrates using both major and minor formulation component step changes to determine the system response using either Near Infrared Spectroscopy or process parameters. These options provide development flexibility to determine the system’s material residence time earlier in the development process and more cost...
05. March 2018
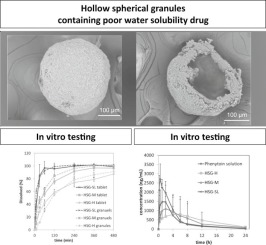
Water-soluble polymers with high viscosity are frequently used in the design of sustained-release formulations of poorly water-soluble drugs to enable complete release of the drug in the gastrointestinal tract. Tablets containing matrix granules with a water-soluble polymer are preferred because tablets are easier to handle and the multiple drug-release units of the matrix granules decreases the influences of the physiological environment on the drug.
26. February 2018
Nine common excipients were examined to determine their ability to cause disproportionation of the HCl salt of a a weakly basic compound. The goal was to determine which excipients were problematic and correlate the results to known properties such as surface pH, slurry pH, or molecular structure. Such a correlation enables a general, simple excipient selection process.
25. November 2017
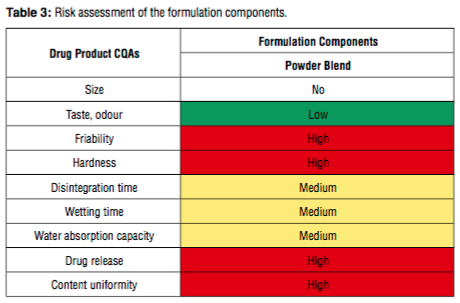
In this study, within the framework of Quality by Design which is a systemati- cally scientific approach which enables to understand and control the production and formulation variables during the process design and development, different parameters in the formulation and production process were detected and criti- cal process parameters and critical material attributes were determined via risk evaluation methods. Then, different oral disintegrating tablet formulations were prepared and tested b