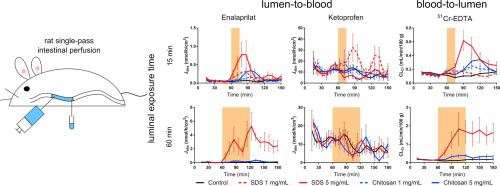
The relevance of the rat single-pass intestinal perfusion model for investigating in
vivo time-dependent effects of absorption-modifying excipients (AMEs) is not fully established. Therefore, the dynamic effect
and recovery of the intestinal mucosa was evaluated based on the lumen-to-blood flux (Jabs) of six model compounds, and the blood-to-lumen
clearance of 51Cr-EDTA (CLCr), during and
after 15- and 60-min mucosal exposure of the AMEs, sodium dodecyl
sulfate (SDS) and chitosan, in
separate experiments. The contribution of enteric neurons on the effect of SDS and chitosan was also evaluated by luminal coadministration of the nicotinic receptor antagonist, mecamylamine. The increases in Jabs and CLCr(maximum and total) during the perfusion experiments were
dependent on exposure time (15 and 60 min), and the concentration of SDS, but not chitosan. The increases in Jabs and CLCrfollowing the 15-min intestinal exposure of both SDS and
chitosan were greater than those reported from an in
vivo rat intraintestinal bolus model. However, the effect in the bolus model could be predicted from the increase of Jabs at the end of the 15-min exposure period, where a
six-fold increase in Jabs was required for
a corresponding effect in the in
vivo bolus model. This illustrates that a rapid and robust effect of the AME is crucial to increase the in
vivointestinal absorption rate before the yet unabsorbed drug in lumen has been transported distally in the intestine. Further, the recovery of the intestinal mucosa
was complete following 15-min exposures of SDS and chitosan, but it only recovered 50% after the 60-min intestinal exposures. Our study also showed that the luminal exposure of AMEs affected the
absorptive model drug transport more than the excretion of 51Cr-EDTA, as Jabs for the drugs was more sensitive than
CLCr at detecting dynamic mucosal AME
effects, such as response rate and recovery. Finally, there appears to be no nicotinergic neural contribution to the absorption-enhancing effect of SDS and chitosan, as luminal administration of
0.1 mM mecamylamine had no effect.