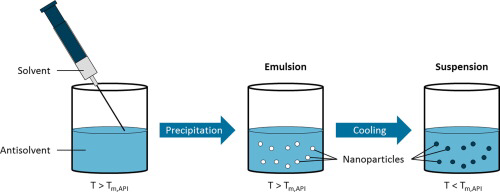
Antisolvent precipitation of poorly water-soluble drugs is a promising formulation technique to synthesize amorphous nanoparticles. The dissolution behavior of these nanoparticles is improved because
of the high specific surface area and the amorphous state, leading to an enhanced bioavailability of the drug molecules. Nevertheless, stabilization of precipitated drug nanoparticles against
agglomeration and recrystallization, which constitutes a key issue for further processing steps, has turned out to be a major challenge. For that reason, the present study presents a synthesis method
to produce long-term stable amorphous ibuprofen nanoparticles via antisolvent precipitation. To reach this goal, a new precipitation method was developed: antisolvent melt precipitation (AMP).
Formulation strategies (e.g. varying fraction of stabilizer) as well as process parameters (e.g. temperature) were under study to estimate their influence on particle size, size distribution,
crystallinity, morphology and stability of synthesized drug nanoparticles.