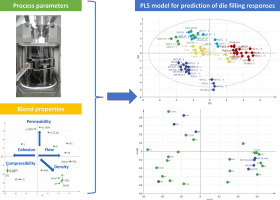
Based on characterization of a wide range of fillers and APIs, thirty divergent blends were composed and subsequently compressed on a rotary tablet press, varying paddle speed and turret speed. The
tablet weight variability was determined of 20 grab samples consisting of each 20 tablets. Additionally, the bulk residence time, ejection force, pre-compression displacement, main compression force,
die fill fraction and feed frame fill fraction were determined during each run. Multivariate data analysis was applied to investigate the relation between the process parameters, blend
characteristics, product and process responses. Blends with metoprolol tartrate as API showed high ejection forces. This behavior could be linked to the high wall friction value of metoprolol
tartrate. The main responses related to the die filling could be predicted via a PLS model based on blend characteristics. Tablet weight variability was highly correlated with the variability on
pre-compression displacement and main compression force. A good predictive model for tablet weight variability was obtained taking the porosity, wall friction angle, flowability, density,
compressibility and permeability into account. Additionally, turret speed and paddle speed were included in the calibration of the model. The applied approach can save resources (material, time)
during early drug product development.