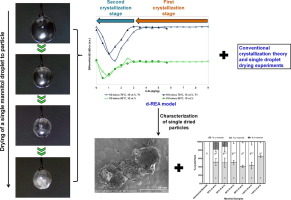
In this paper, we explain the crystallization process of mannitol during convective droplet drying based on the crystallization kinetics calculated from two mathematical models coupled with experimental investigations. A novel differential-reaction engineering approach was developed, correlating the mannitol crystallization behavior to the deviation of droplet drying kinetics at different drying temperatures. The model was compared with a conventional glass transition-based model and the evolutions of droplet saturation state, density and morphology during drying were experimentally determined, to provide a comprehensive analysis on the crystallization of mannitol as droplet drying progressed. Two crystallization stages were identified. Mannitol solids firstly nucleated and precipitated at droplet surface, and the crystallization kinetics was similar at different drying temperatures (70, 90 and 110 °C). The removal of residual moisture at the second stage was dependent on the drying temperature, with lower temperatures of 70 and 90 °C exhibiting an extended crystallization period associated with slow release of moisture. The morphological and polymorphism analyses on dried mannitol particles suggested a possible growth of stable or metastable polymorphs during the prolonged crystallization event. The results for the first time unveil the unique in-drying crystallization behavior of mannitol droplets, providing fundamental information needed in industrial spray drying for controlling the crystal form of mannitol-based drugs. The proposed mathematical and experimental approaches can be utilized in analyzing other crystallization-prone materials.