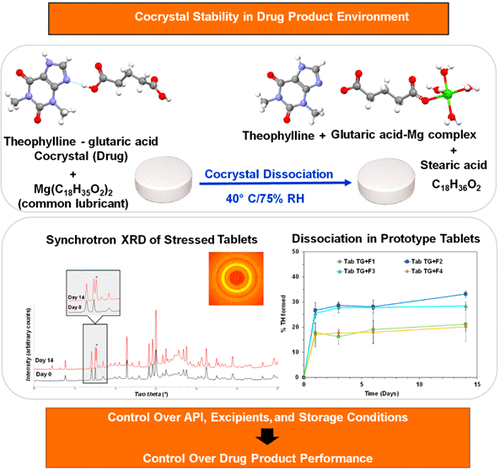
Tablets containing a theophylline–glutaric acid (TG) cocrystal dissociated rapidly forming crystalline theophylline (20–30%), following storage at 40 °C/75% RH for 2 weeks. Control tablets of TG cocrystal containing no excipients were stable under the same conditions. The dissociation reaction was water-mediated, and the theophylline concentration (the dissociation product), monitored by synchrotron X-ray diffractometry, was strongly influenced by the formulation composition. Investigation of the binary compacts of the TG cocrystal with each excipient revealed the influence of excipient properties (hydrophilicity, ionizability) on cocrystal stability, providing mechanistic insights into a dissociation reaction. Ionizable excipients with a strong tendency to sorb water, for example, sodium starch glycolate and croscarmellose sodium, caused pronounced dissociation. Microcrystalline cellulose (MCC), while a neutral but hydrophilic excipient, also enabled solution-mediated cocrystal dissociation in intact tablets. Magnesium stearate, an ionizable but hydrophobic excipient, interacted with the cocrystal to form a hygroscopic product. The interaction is believed to be initiated in the disordered cocrystal–excipient particle interface. In contrast, the cocrystal was stable in the presence of lactose, a neutral excipient with no tendency to sorb water. The risk of unintended cocrystal dissociation can be mitigated by avoiding contact with water both during processing and storage.
More on molecular pharmaceutics
#excipients