"Common defects in tablet film coating can be minimized by effective design of the tablet core and the coating process." Stuart C. Porter in Pharmaceutical Technology
Film coatings on pharmaceutical tablets provide a broad range of functionalities, and the coating formulation and process are complex, with many variables. Figure 1 shows the variables associated with a pan-coating process, for example. All of these factors can potentially influence critical quality attributes of the final coated product.
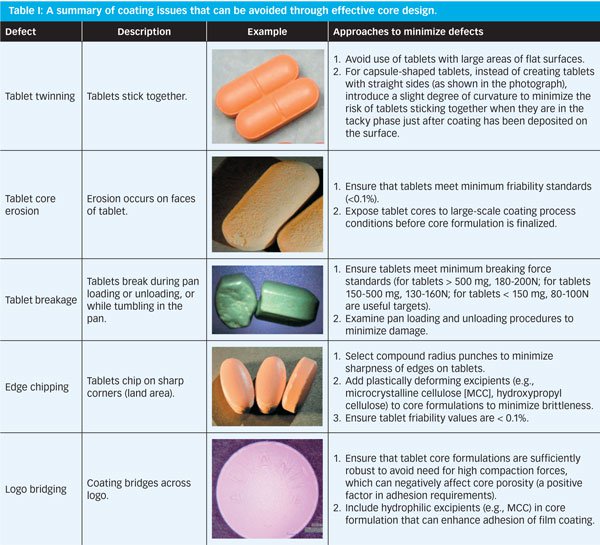
Film-coated products seem to exhibit more than their fair share of defects. Although many film-coating problems tend to be visual or cosmetic defects that may not negatively impact product efficacy, their existence detracts from the perception of overall product quality and may affect consumer/patient confidence. Many common problems with film-coated products and processes are understood and solutions have been discussed (1–4). Most of these problems can be avoided if a scientifically valid and proactive approach is taken when designing formulations and processes.
Generally speaking, the key elements of any film-coated product are formulation and design of the core (to which the coating is applied), the coating formulation, and the coating process. In many cases, all three of these elements, in combination, play a key role in the presence or absence of coating defects.
Problems associated with film-coated products may be classified as:
- Visual defects
- Issues associated with product functionality (e.g., drug release behavior)
- Concerns with product stability
- Factors affecting manufacturing costs.
Identifying visual defects (and the extent to which they occur) can be relatively simple, and there are proactive approaches to avoiding them. Product functionality and stability, however, present a more complex situation, because the existence of the problem is typically not apparent until after the coating process has been concluded, and sample selection for testing purposes often effectively precludes assessing the magnitude of the problem.
Preventing problems is better than troubleshooting afterwards, because regulatory issues (e.g., after a product has been commercialized) may limit troubleshooting solutions. Indeed, the intent of quality-by-design practices is to investigate all aspects of the product development process and identify critical quality attributes and critical process parameters. This article provides an overview of issues that should be considered when designing film-coated products. Pan coating of tablets is used as an example, but many of the assertions are relevant to film coating in general.
Continue on pharmtech.com or in the pdf version
By Stuart C. Porter, Ashland Specialty Ingredients
Pharmaceutical Technology
Volume 40, Issue 2, pg 42–45