In part I of this series, I wrote about the financial unsustainability of antibiotics development. Revenues and profits of the new therapies launched since 2010 do not match the costs of development and thus most large pharmaceutical companies and biotech investors have stopped supporting antibiotic development.
Fixing the broken business system will require a concerted effort by all stakeholders: payers, physicians, regulators, and the biopharmaceutical industry on a global scale. In this part of the series, I will focus on the innovation of the biotech community.
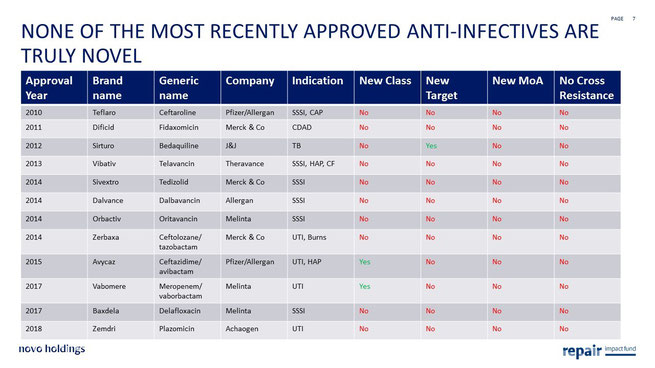
My central hypothesis is that novel innovative therapeutics (novel drug classes with novel mechanisms of action) will not only be differentiated clinically but will also fare better commercially. Unfortunately, the development of these types of drugs has been a rarity in recent years.
The WHO published in 2017 a report outlining the late stage antimicrobial clinical development pipeline. As can be seen on the following figures, none of the products approved since 2010 and only few of the products in phase 2 and 3 are truly novel as defined by being a new molecular class, with a new target, operating through a new mechanism of action and therefore without expected cross resistance to existing drugs on the market.