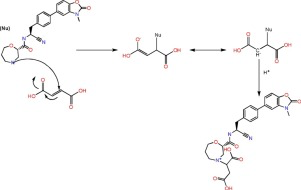
During compatibility study of the AZD7986 project, a peak of 3 area% at the tail (RRT 1.03) of the active pharmaceutical ingredient (API) was discovered for all tablets containing sodium stearyl fumarate (PRUV) under humid condition (e.g. 50 °C/75% RH), regardless of choice of disintegrant or filler combination. The degradant was needed to be identified to understand the corresponding reaction mechanism and help the final formulation design. Structure elucidation was therefore done by analysis using high resolution mass spectrometry. The degradant was found to be a Michael addition product of the API and fumaric acid. Reaction between deuterated fumaric acid and the API was carried to confirm the proposed structure and reaction mechanism. Fumaric acid was a degradant product of PRUV in the presence of other excipients, revealed by the stability study. The Michael addition reaction needs facilitation by water and basic conditions. The result from this study should serve as a precaution note for projects using PRUV as one of excipients where the API could act as a nucleophile. In such cases the microenvironment should be optimised to minimize the reaction, such as pH adjustment and incorporating protection from moisture.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact