Novel excipients are indispensable in development of modern, advanced drug delivery systems and biotechnology-derived drugs. Although numerous novel excipients are developed for pharmaceutical use,
they are not frequently seen in medicinal products due to the strict regulatory requirements and perception that their use makes new product evaluation more complex with risk of delays in the
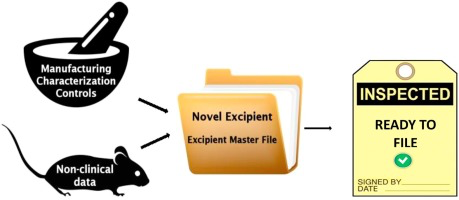
approval process. Regulators regard novel excipients as new substances and whenever new excipient is used in a formulation it must be subjected to full evaluation, similarly to the one required for
new active substance. Consequently, the amount of information required in support of the regulatory approval (i.e. marketing authorization) is much more complex and comprehensive than for established
excipients. This short review provides an insight into the use of novel excipients in medicinal products approved in the European Union. In addition, barriers and challenges in development of novel
excipients are being discussed as well as means to overcome those barriers.