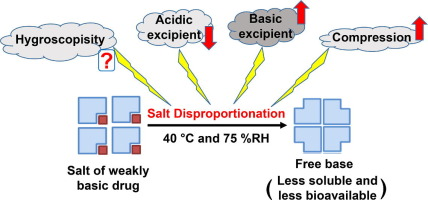
Excipients are crucial components of most pharmaceutical formulations. In the case of a solid oral dosage formulation containing the salt form of a weakly ionizable drug, excipient selection is
critical, as some excipients are known to cause salt disproportionation (conversion of salt to the free form). Therefore, robust formulation design necessitates an in-depth understanding of the
factors impacting salt disproportionation during processing or storage as this can negatively impact product quality and performance. To date, there is an incomplete understanding of key excipient
properties influencing salt disproportionation. Specifically, the potential roles of amorphous excipient glass transition temperature and excipient hygroscopicity, if any, on salt disproportionation
are still not well understood. Furthermore, the relationship between the compression and the extent of salt disproportionation is an unknown factor. Herein, by utilizing various grades of
polyvinylpyrolidone (PVP), its copolymer, copovidone (PVPVA), and magnesium stearate, a systematic investigation on disproportionation was performed using pioglitazone HCl as a model salt of a weak
base. It was observed that there was a poor correlation between excipient hygroscopicity and the rate and extent of disproportionation. However, powder compression into compacts enhanced the rate and
extent of disproportionation. This work focused on disproportionation of the salt of a weak base, as basic drugs are more prevalent, however, salts of weak acids may have similar tendencies under
relevant conditions. The knowledge gained from this study will help in understanding the role of various excipients with respect to salt disproportionation, paving the way for designing stable salt
formulations.