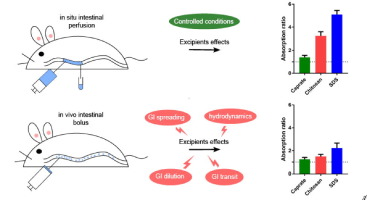
Pharmaceutical excipients that may affect gastrointestinal (GI) drug absorption are called critical pharmaceutical excipients (CPEs), or absorption-modifying excipients (AMEs) if they act by altering
the integrity of the intestinal epithelial cell membrane. Some of these excipients increase intestinal permeability, and subsequently the absorption and bioavailability of the drug. This could have
implications for both the assessment of bioequivalence and the efficacy of the absorption-enhancing drug delivery system. The absorption-enhancing effects of AMEs/CPEs with different mechanisms
(chitosan, sodium caprate, sodium dodecyl sulfate (SDS)) have previously been evaluated in the rat single-pass intestinal perfusion (SPIP) model. However, it remains unclear whether these SPIP data
are predictive in a more in vivo like model.
The same excipients were in this study evaluated in rat and dog intraintestinal bolus models. SDS and chitosan did exert an absorption-enhancing effect in both bolus models, but the effect was
substantially lower than those observed in the rat SPIP model. This illustrates the complexity of the AME/CPE effects, and indicates that additional GI physiological factors need to be considered in
their evaluation. We therefore recommend that AME/CPE evaluations obtained in transit-independent, preclinical permeability models (e.g. Ussing, SPIP) should be verified in animal models better able
to predict in vivo relevant GI effects, at multiple excipient concentrations.