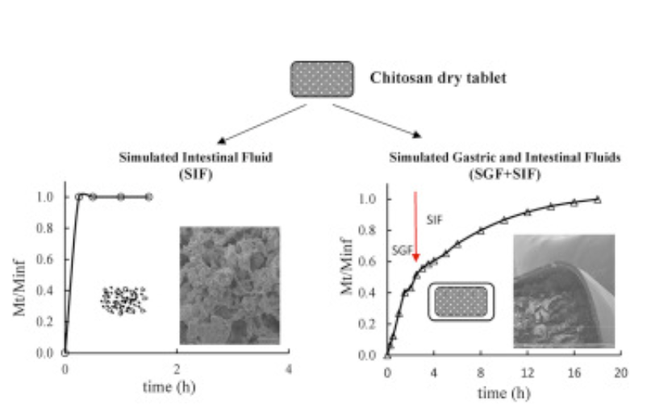
This study focuses on the behavior of chitosan (CHI) and its polyelectrolyte complexes with carboxymethyl starch (CMS) used as monolithic matrices with acetaminophen as drug tracer. Two different chitosan grades were tested alone or associated in various ratios with CMS as excipients for tablets obtained by direct compression. The degree of deacetylation (DDA) of CHI, estimated from 1H NMR and FTIR data, was correlated with X-ray diffraction and scanning electron microscopy (SEM) to evaluate structural organization of the monolithic matrices. In vitro drug dissolution assays showed major differences in CHI kinetic profiles between tablets exposed to acidic medium for 2h (to mimick gastric passage) prior to dissolution in simulated intestinal fluid (SIF), and those administered directly to SIF. Prior exposure to acidic SGF conducted to longer dissolution profiles (release completed after 16 h) and preservation of tablet shape, whereas tablets directly incubated in SIF were rapidly disintegrated. The improved properties of chitosan matrices exposed to SGF may be related to an outer compact coating layer (visible in SEM). The effect of self-stabilization of chitosan in acidic medium was compared to that due to formation of polyelectrolyte complexes (PEC) in co-processed polymeric systems (CHI:CMS). The self-formed membrane following exposure to gastric acidity appears to help maintaining tablet integrity and allows higher drug loading, recommending CHI and its complexes with CMS as excipients for drug delivery.
PDF Download of the full article from Bioactive Materials Volume 3, Issue 3, September 2018, Pages 334–340 - see below.