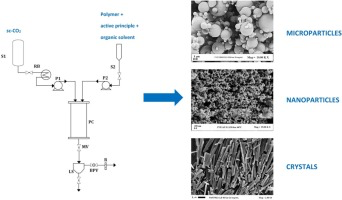
Supercritical antisolvent precipitation (SAS) has been successfully used to produce microparticles and nanoparticles of controlled size and distribution either as a single precipitates or by coprecipitation of two or more compounds. SAS coprecipitation process has produced different particles morphologies and, differently from the single compound SAS precipitation, process mechanisms involved have never been elucidated and the effectiveness of the technique has been verified only in some cases.
In this work, the mechanisms proposed in SAS coprecipitation are critically discussed and general indications about coprecipitation efficiency are given, based on several experimental evidences and on the possible underlying nucleation, growth and drying mechanisms.
The most effective and reliable SAS coprecipitation resulted from the formation of microdroplets and their subsequent drying.