Surfactants are common stabilizers, often added to biopharmaceuticals formulations, but the mechanisms at the basis of their activity are unclear. The aim of this work is to provide insight into the molecular factors underlying surfactant effectiveness as protectants.
Methods
Molecular Dynamics simulations of human growth hormone (hGH) in the presence of Tween 20 were performed. The effect of Tween 20 was compared with the activity of commonly used protectants, such as the disaccharides.
Results
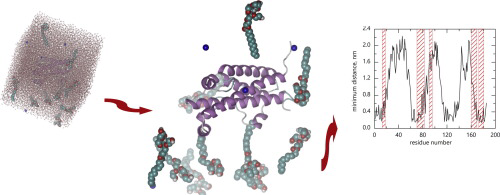
We found that Tween 20 could prevent the self-association of hGH, leading to the formation of a protein-surfactant complex. In the case of unfolded hGH, surfactant molecules were oriented with their hydrophilic head in the direction of the protein hydrophobic patches. This created a highly unstable situation, fostering refolding. In the case of native hGH, Tween 20 molecules oriented with their lipophilic groups in the direction of the protein surface. This thermodynamically stabilized the native conformation, preventing unfolding.
Conclusions
We found that the ability of surfactants to foster protein refolding is related to their amphiphilic nature, and, more specifically, to their specific orientation with respect to the protein surface. A molecular mechanism explaining surfactant activity is proposed, which could provide direction for improvements in biopreservation.