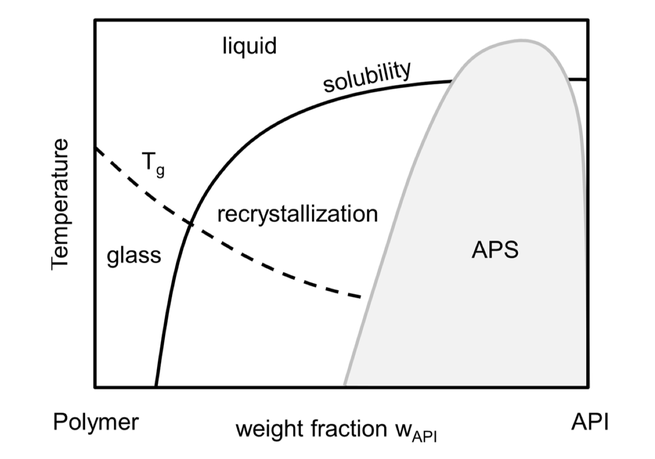
The long-term stability of pharmaceutical formulations of poorly-soluble drugs in
polymers determines their bioavailability and therapeutic applicability. However, these formulations
do not only often tend to crystallize during storage, but also tend to undergo unwanted
amorphous-amorphous phase separations (APS). Whereas the crystallization behavior of APIs in
polymers has been measured and modeled during the last years, the APS phenomenon is still
poorly understood. In this study, the crystallization behavior, APS, and glass-transition temperatures
formulations of ibuprofen and felodipine in polymeric PLGA excipients exhibiting different ratios of
lactic acid and glycolic acid monomers in the PLGA chain were investigated by means of hot-stage
microscopy and DSC. APS and recrystallization was observed in ibuprofen/PLGA formulations,
while only recrystallization occurred in felodipine/PLGA formulations. Based on a successful
modeling of the crystallization behavior using the Perturbed-Chain Statistical Associating Fluid
Theory (PC-SAFT), the occurrence of APS was predicted in agreement with experimental findings.