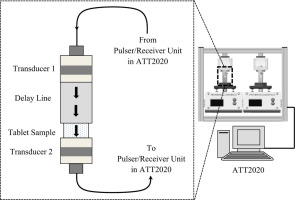
Currently, the compressed tablet and its oral administration is the most popular drug delivery modality in medicine. The accurate porosity and tensile strength characterization of a tablet design is vital for predicting its performance such as disintegration, dissolution, and drug-release efficiency upon administration as well as ensuring its mechanical integrity. In current work, a non-destructive contact ultrasonic approach and an associated testing procedure are presented and employed to quantify and relate the acoustically extracted mechanical properties of pharmaceutical compacts to direct porosity and tensile strength measurements. Based on a comprehensive set of experimental data, it is demonstrated how strongly the acoustic wave propagation is modulated and correlated to the tablet porosity and tensile strength of a compact made using spray-dried lactose and microcrystalline cellulose with varying mixture ratios. The effect of mixing ratio on the porosity and tensile strength on the resulting compacts is quantified and, with the acoustic experimental data, mixing ratio is related to the compact ultrasonic characteristics. The ultrasonic techniques provide a rapid, non-destructive means for evaluating compacts in formulation development and manufacturing. The presented approach and data could find critical applications in continuous tablet manufacturing, its real-time quality monitoring, as well as minimizing batch-to-batch quality variations.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact