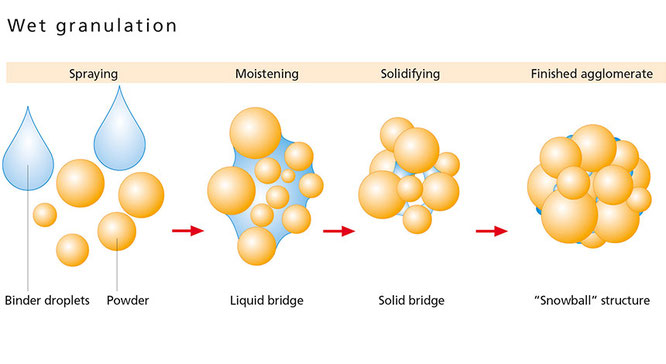
Granulation is an integral step during pharmaceutical manufacturing of solid doses, which is usually followed by unit operations such as drying, milling, tableting and coating. Granulation is typically carried out to alleviate problems in powder handling, content non-uniformity, segregation and poor flow properties of powders. However, previous work carried out by Oka et. al. (Oka, Emady et al. 2015) points out that sometimes a wet granulation process might fail to give us a robust product, especially in case of a low dose drug product. Multiple factors such as segregation, wettability properties of formulation ingredients and active pharmaceutical ingredient (API) solubility in the binder solution were found to impact the distribution and consequent availability of the drug in the finished dosage form. The focus of this work is to understand the effect of the solubility of an API on its distribution within granule cores and across granule size classes upon drying. When a wet granule containing a water-soluble API is dried, it is likely that the dissolved API will migrate towards the periphery of the granule as the solvent evaporates. Consequently, the migrated active can deposit itself on the outer crust of the granule upon recrystallization from the evaporating binder solvent. Subsequent powder handling may lead to attrition of this deposited active, thereby creating super-potent fines and resulting in severe non-homogeneity and product losses (Oka, Emady et al. 2015). In the case of pharmaceutical granulation, a greater extent of API migration can not only compromise the structural integrity of the granules (Poutiainen, Honkanen et al. 2012) but also lead to poor flow and incomplete granulation resulting in content nonuniformity. This thesis investigates the extent of drug (active) migration in granules made via high shear wet granulation subject to the viscosity of the binder solution, particle size of the excipient and granule porosity. X-ray micrtomography was used to analyze the spatial distribution of the recrystallized API. Due to the complexity of a qualitative comparison between granules having different sizes, shape and porosities, a quantification technique that is independent of these differences is warranted. The extent of capillary migration in the resulting granules was analyzed by dividing the μ-CT images into conical sections and quantifying the distribution of the active across these conical cross-sections. Computational tools have been used to define a dimensionless radial distribution function (RDF) to quantify the spatial distribution of the active ingredient in granules produced under different processing conditions. Statistical analysis was performed to quantify the extent of aforementioned variables on the extent of migration. Thus, a comprehensive investigation into the causes of drug migration was carried out in this study to ascertain which factors or combination of factors have the most prominent effect on the extent of migration of a water soluble API during granule drying. Hence, this study aims to identify the optimal operating conditions and drying parameters to design a robust wet granulation process and subsequently address content non-uniformity issues in pharmaceutical processes.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact