By Drug Development & Delivery
Issue: March 2018, Posted Date: 2/27/2018
This past year marked a milestone in the pharma industry when Aprecia Pharmaceuticals’ Spritam® (levetiracetam) tablets became the first FDA-approved prescription drug product manufactured using 3D printing technology.
“As we explored potential applications for our 3D printing technology in prescription drug products, it was important that we identified disease areas with a real need for patient-friendly forms of medication,” said Don Wetherhold, CEO of Aprecia, in a printed statement.
Spritam is formulated with Aprecia’s proprietary ZipDose® Technology, which combines the precision of 3D printing and formulation science to produce rapidly disintegrating formulations of medications. An inkjet printing process produces the porous water-soluble drug layer by layer by printing aqueous fluid onto layers of powdered medication, without compression or traditional molding techniques, explains Sonia Mannan, Commissioning Editor, Journal of 3D Printing in Medicine. She says researchers from University College London’s School of Pharmacy are using hot-melt extrusion to 3D print different shaped drugs to explore the connection between drug geometry and drug release.
“It’s exciting to see the ways 3D printing is being used in the medical environment,” says Laura Dormer, Editorial Director for Future Medicine, publishers of Journal of 3D Printing in Medicine. “To date, this has predominantly involved printing of plastics and metals, such as surgical planning, prosthetics, or reconstructive surgery. Surgeons are now able to 3D print accurate models of their patients’ organs, allowing them to plan complex procedures with a higher degree of confidence than with imaging alone. 3D printing has also been used to create tailored bone inserts for use in complex facial reconstructive surgery. 3D printing of pharmaceuticals is less advanced, but offers an exciting opportunity for the future, as does the possibility of printing with organic materials (bioprinting) for use in regenerative medicine.”
PERSONALIZED MEDICINE THROUGH 3D PRINTING
One area of 3D printing that also holds exciting promise is in personalized medicine. As new drugs are developed that have increasing potency and differential effects within populations, there is a need to consider new manufacturing methods and novel supply chains to realize the paradigm of personalised medicines. 3D printing offers the possibility of creating a personalized medicine system through automated control over drug dose and is suitable for both low and high drug concentrations.
FabRx is a research-driven specialist biotech company, focused on developing 3D printing technology for fabricating pharmaceutical dosage forms and medical devices. FabRx was founded in 2014 as a spin-out from University College London (UCL) and operates from the UCL School of Pharmacy, giving the company access to the latest equipment in 3D printing and analytical technology. The company also develops formulations and printing technologies for third-party organizations.
“Our team has a wealth of experience in all aspects of oral drug formulation and knowledge of the challenges of bringing new medicines through the (often complex) regulatory processes of the pharma sector,” says Prof. Simon Gaisford, Printing Technology Director at FabRx. “We are developing printable formulations for personalized drug delivery, and as part of this we are adapting printing technology.”
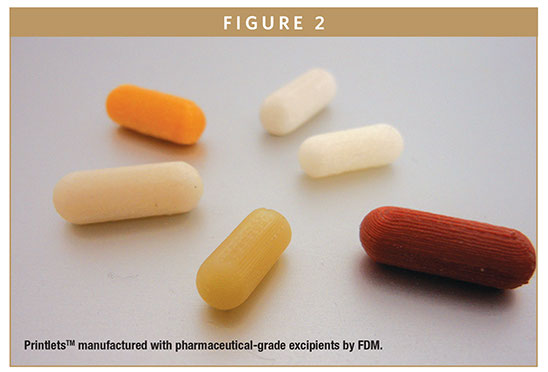
FabRx has specialist experience in using all the 3D printing technologies that can be used in pharmaceutics, but focuses particularly on fused deposition modelling (FDM), powder bed printing, and stereolithography (SLA) to formulate 3D printed medicines. Its proprietary 3D printed medicines are called PrintletsTM.
“These are novel drug formulations that achieve personalized dose and controlled drug release profiles that can be tailored to the individual needs of each drug and that cannot readily be prepared by other manufacturing methods,” says Dr. Alvaro Goyanes, Director of Development at FabRx. “Printlets technology offers a proprietary platform technology to formulate and manufacture 3D printed medicines with nearly any drug compound with a high control of the dose strength (the main requirement for personalization). FabRx technology allows manufacturing of Printlets with a diverse range of shapes, sizes, colors, textures, and flavors to make them more attractive to various patient groups, particularly the young or the elderly, facilitating compliance of the treatment.” Additionally, he says it is possible to incorporate multiple drugs within one Printlet, to make fixed-dose combinations.
Selection of the excipients or the dosage form design means the time of release and/or the release kinetics of each active can be finely tuned. FabRx can fabricate Printlets with a range of GRAS pharmaceutical excipients. Proper selection of excipients allows FabRx to design Printlets possessing any desired drug release profile, ranging from immediate release to sustained and delayed release (including zero-order).
“3D printing could be used to customize specific drugs or drug cocktails based on an individual, but if you want to take it another step further, we could see a future where diagnostic and genomic sequencing technologies are interoperable with the 3D printer to automate calibration and production of the drug for a given individual,” says Reenita Das, Transformational Health Partner and Senior Vice President, at Frost & Sullivan. “One-size-fits-all is not a model that is not ideal for healthcare where you have such a wide spectrum of individuals based on age, height, weight, gender, ethnicity, hereditary traits, co-morbidities, disabilities, etc. The core value driver of 3D printing is the ability to allow for mass customization at scale. 3D printed drugs offer the ability to individualize dosing, tailor drug release profiles, drug combinations, and optimize the supply chain for certain hard-to-get therapeutics.”