Abstract
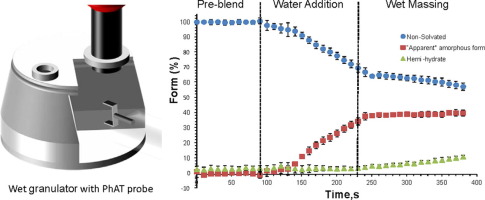
Form changes during drug product processing can be a risk to the final product quality in terms of chemical stability and bioavailability. In this study, online Raman spectroscopy was used to monitor the form changes in real time during high shear wet granulation of Compound A, a highly soluble drug present at a high drug load in an extended release formulation. The effect of water content, temperature, wet massing time and drying technique on the degree of drug transformation were examined. A designed set of calibration standards were employed to develop quantitative partial least square regression models to predict the concentration of each drug form during both wet granulation and the drying process. Throughout all our experiments we observed complex changes of the drug form during granulation, manifest as conversions between the initial non-solvated form of Compound A, the hemi-hydrate form and the “apparent” amorphous form (dissolved drug). The online Raman data demonstrate that the non-solvated form converts to an “apparent” amorphous form (dissolved drug) due to drug dissolution with no appearance of the hemi-hydrate form during water addition stage. The extent of conversion of the non-solvated form was governed by the amount of water added and the rate of conversion was accelerated at higher temperatures. Interestingly, in the wet massing zone, the formation of the hemi-hydrate form was observed at a rate equivalent to the rate of depletion of the non-solvated form with no change in the level of the “apparent amorphous” form generated. The level of hemi-hydrate increased with an increase in wet massing time. The drying process had a significant effect on the proportion of each form. During tray drying, changes in drug form continued for hours. In contrast fluid bed drying appeared to lock the final proportions of drug form product attained during granulation, with comparatively small changes observed during drying. In conclusion, it was possible to simultaneously monitor the three forms in real time during wet granulation and drying using online Raman spectroscopy. The results regarding the effect of process parameters on the degree of transformation are critical for designing a robust process that ensures a consistent form in the final drug product.
Conclusion
The evolution of the three forms of HIV attachment inhibitor compound “A” was successfully monitored during wet granulation and drying using online Raman spectroscopy. The online Raman data
demonstrate that the non-solvated form converts to an “apparent” amorphous form (dissolved drug) due to drug dissolution with no appearance of the hemi-hydrate form during
water addition stage. The extent of conversion of the non-solvated form was governed by the amount of water added and the rate of conversion was accelerated at higher temperatures.
Interestingly, in the wet massing zone, the formation of the hemi-hydrate form was observed at a rate equivalent to the rate of depletion of the non-solvated form with no change in the
level of the “apparent amorphous” form generated. The level of hemi-hydrate increased with an increase in wet massing time. The pathway for the generation of the hemi-hydrate is unclear but
is believed to be either from re-crystallization of the dissolved drug (apparent amorphous form) or from a solid state transition of the non-solvated drug. The different forms attained at the end
of granulation either get trapped (fluid bed drying) or continue to change (tray drying) demonstrating that the drying rate influences the degree of form changes. The knowledge gained from this
study around complexity of form transformation (rate and extent) relative to process parameter ranges indicated that wet granulation posed additional risks leading to selection of an
alternate manufacturing process for future clinical stages.